MCT4 blockade increases the efficacy of immune checkpoint blockade
- PMID: 37880183
- PMCID: PMC10603342
- DOI: 10.1136/jitc-2023-007349
MCT4 blockade increases the efficacy of immune checkpoint blockade
Abstract
Background & aims: Intratumoral lactate accumulation and acidosis impair T-cell function and antitumor immunity. Interestingly, expression of the lactate transporter monocarboxylate transporter (MCT) 4, but not MCT1, turned out to be prognostic for the survival of patients with rectal cancer, indicating that single MCT4 blockade might be a promising strategy to overcome glycolysis-related therapy resistance.
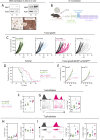
Methods: To determine whether blockade of MCT4 alone is sufficient to improve the efficacy of immune checkpoint blockade (ICB) therapy, we examined the effects of the selective MCT1 inhibitor AZD3965 and a novel MCT4 inhibitor in a colorectal carcinoma (CRC) tumor spheroid model co-cultured with blood leukocytes in vitro and the MC38 murine CRC model in vivo in combination with an antibody against programmed cell death ligand-1(PD-L1).
Results: Inhibition of MCT4 was sufficient to reduce lactate efflux in three-dimensional (3D) CRC spheroids but not in two-dimensional cell-cultures. Co-administration of the MCT4 inhibitor and ICB augmented immune cell infiltration, T-cell function and decreased CRC spheroid viability in a 3D co-culture model of human CRC spheroids with blood leukocytes. Accordingly, combination of MCT4 and ICB increased intratumoral pH, improved leukocyte infiltration and T-cell activation, delayed tumor growth, and prolonged survival in vivo. MCT1 inhibition exerted no further beneficial impact.
Conclusions: These findings demonstrate that single MCT4 inhibition represents a novel therapeutic approach to reverse lactic-acid driven immunosuppression and might be suitable to improve ICB efficacy.
Keywords: Drug Therapy, Combination; Immune Checkpoint Inhibitors; Immunologic Surveillance; Lymphocytes, Tumor-Infiltrating; Metabolism; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The study was conducted in close collaboration with Merck. A.S-H., T.H., and C.H. are employees of Merck. R.F. is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. S.R. was an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA at the time the research was conducted.
Figures




References
-
- Walenta S, Wetterling M, Lehrke M, et al. . High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human Cervical cancers. Cancer Res 2000;60:916–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
