Enhanced protective efficacy of a thermostable RBD-S2 vaccine formulation against SARS-CoV-2 and its variants
- PMID: 37880298
- PMCID: PMC10600342
- DOI: 10.1038/s41541-023-00755-2
Enhanced protective efficacy of a thermostable RBD-S2 vaccine formulation against SARS-CoV-2 and its variants
Abstract
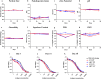
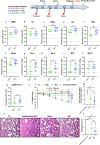
With the rapid emergence of variants of concern (VOC), the efficacy of currently licensed vaccines has reduced drastically. VOC mutations largely occur in the S1 subunit of Spike. The S2 subunit of SARS-CoV-2 is conserved and thus more likely to elicit broadly reactive immune responses that could improve protection. However, the contribution of the S2 subunit in improving the overall efficacy of vaccines remains unclear. Therefore, we designed, and evaluated the immunogenicity and protective potential of a stabilized SARS-CoV-2 Receptor Binding Domain (RBD) fused to a stabilized S2. Immunogens were expressed as soluble proteins with approximately fivefold higher purified yield than the Spike ectodomain and formulated along with Squalene-in-water emulsion (SWE) adjuvant. Immunization with S2 alone failed to elicit a neutralizing immune response, but significantly reduced lung viral titers in mice challenged with the heterologous Beta variant. In hamsters, SWE-formulated RS2 (a genetic fusion of stabilized RBD with S2) showed enhanced immunogenicity and efficacy relative to corresponding RBD and Spike formulations. Despite being based on the ancestral Wuhan strain of SARS-CoV-2, RS2 elicited broad neutralization, including against Omicron variants (BA.1, BA.5 and BF.7), and the clade 1a WIV-1 and SARS-CoV-1 strains. RS2 elicited sera showed enhanced competition with both S2 directed and RBD Class 4 directed broadly neutralizing antibodies, relative to RBD and Spike elicited sera. When lyophilized, RS2 retained antigenicity and immunogenicity even after incubation at 37 °C for a month. The data collectively suggest that the RS2 immunogen is a promising modality to combat SARS-CoV-2 variants.
© 2023. Springer Nature Limited.
Conflict of interest statement
A provisional patent application has been filed for the RS2 formulations described in this manuscript. R.V., N.M., and R.S. are inventors. R.V. is a co-founder of Mynvax, R.S., S.B.J., N.J., M.B., and S.P. are employees of Mynvax Private Limited. Other authors declare that they have no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

