Normative spatiotemporal fetal brain maturation with satisfactory development at 2 years
- PMID: 37880365
- PMCID: PMC10620088
- DOI: 10.1038/s41586-023-06630-3
Normative spatiotemporal fetal brain maturation with satisfactory development at 2 years
Abstract
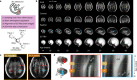
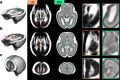
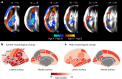
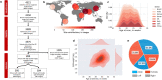
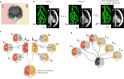
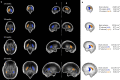
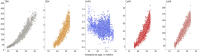
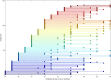
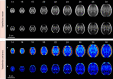
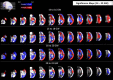
Maturation of the human fetal brain should follow precisely scheduled structural growth and folding of the cerebral cortex for optimal postnatal function1. We present a normative digital atlas of fetal brain maturation based on a prospective international cohort of healthy pregnant women2, selected using World Health Organization recommendations for growth standards3. Their fetuses were accurately dated in the first trimester, with satisfactory growth and neurodevelopment from early pregnancy to 2 years of age4,5. The atlas was produced using 1,059 optimal quality, three-dimensional ultrasound brain volumes from 899 of the fetuses and an automated analysis pipeline6-8. The atlas corresponds structurally to published magnetic resonance images9, but with finer anatomical details in deep grey matter. The between-study site variability represented less than 8.0% of the total variance of all brain measures, supporting pooling data from the eight study sites to produce patterns of normative maturation. We have thereby generated an average representation of each cerebral hemisphere between 14 and 31 weeks' gestation with quantification of intracranial volume variability and growth patterns. Emergent asymmetries were detectable from as early as 14 weeks, with peak asymmetries in regions associated with language development and functional lateralization between 20 and 26 weeks' gestation. These patterns were validated in 1,487 three-dimensional brain volumes from 1,295 different fetuses in the same cohort. We provide a unique spatiotemporal benchmark of fetal brain maturation from a large cohort with normative postnatal growth and neurodevelopment.
© 2023. The Author(s).
Conflict of interest statement
J.A.N. and A.T.P. are Senior Scientific Advisers of Intelligent Ultrasound but the company has no financial or intellectual property links with the research described in this paper and the described work is entirely based on their academic work. We declare that all the other authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures








References
-
- Villar J, et al. The satisfactory growth and development at 2 years of age of the INTERGROWTH-21st Fetal Growth Standards cohort support its appropriateness for constructing international standards. Am. J. Obstet. Gynecol. 2018;218:S841–S854 e842. doi: 10.1016/j.ajog.2017.11.564. - DOI - PMC - PubMed

