Tisagenlecleucel vs. historical standard of care in children and young adult patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia
- PMID: 37880478
- PMCID: PMC10681894
- DOI: 10.1038/s41375-023-02042-4
Tisagenlecleucel vs. historical standard of care in children and young adult patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia
Abstract
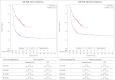
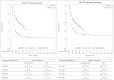
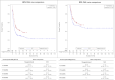
In the absence of randomized controlled trials comparing tisagenlecleucel vs. standard of care (SOC) in pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia (r/r ALL), the objective was to compare the efficacy of tisagenlecleucel with historical controls from multiple disease registries using patient-level adjustment of the historical controls. The analysis is based on patient-level data of three tisagenlecleucel studies (ELIANA, ENSIGN and CCTL019B2001X) vs. three registries in Germany/Austria. Statistical analyses were fully pre-specified and propensity score weighting of the historical controls by fine stratification weights was used to adjust for relevant confounders identified by systematic literature review. Results showed high comparability of cohorts after adjustment with absolute SMD ≤ 0.1 for all pre-specified confounders and favorable outcomes for tisagenlecleucel compared to SOC for all examined endpoints. Hazard ratios for OS(Intention to treat)ITT,adjusted, EFS(Full analysis set)FAS,naïve and RFSFAS,naïve were 0.54 (95% CI: 0.41-0.71, p < 0.001), 0.67 (0.52-0.86, p = 0.001) and 0.77 (0.51-1.18, p = 0.233). The OSITT, adjusted, EFSFAS,naïve and RFSFAS,naive survival probability at 2 years was 59.49% for tisagenlecleucel vs. 36.16% for SOC population, 42.31% vs. 30.23% and 59.60% vs. 54.57%, respectively. Odds ratio for ORRITT,adjusted was 1.99 (1.33-2.97, p < 0.001). Results for OS and ORR were statistically significant after adjustment for confounders and provide evidence supporting a superiority of tisagenlecleucel in r/r ALL given the good comparability of cohorts after adjustment for confounders.
© 2023. The Author(s).
Conflict of interest statement
This study was funded by Novartis Pharma GmbH. Medical writing was provided by Daniela Skalt, Stephanie Sussmann and Fabian Berkemeier. Statistical analyses were provided by Heidi Kulas and Guido Schiffhorst. These are employed by IGES Institut GmbH, which received financial support from Novartis Pharma GmbH for this research project. Katja Jäschke, Corinna Templin, Ulli Jeratsch and Daniela Kosmides are employees from Novartis GmbH and Etienne Jousseaume from Novartis Pharma AG which sponsored the studies ELIANA, ENSIGN and CCTL019B2001X.
Figures


References
-
- Deutsche Gesellschaft für Hämatologie und Onkologie (DGHO). Akute lymphatische Leukämie (ALL); 2022 [cited 2022 Jul 6]. Available from: https://www.onkopedia.com/de/onkopedia/guidelines/akute-lymphatische-leu....
-
- Kraywinkel K, Spix C. Epidemiologie akuter Leukämien in Deutschland. Der Onkol. 2017;23:499–503. doi: 10.1007/s00761-017-0249-z. - DOI
-
- Peters C, Dalle J-H, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol: Off J Am Soc Clin Oncol. 2021;39:295–307. doi: 10.1200/JCO.20.02529. - DOI - PMC - PubMed
-
- Peters C, Schrappe M, Stackelberg AV, Schrauder A, Bader P, Ebell W, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33:1265–74. doi: 10.1200/JCO.2014.58.9747. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

