Adverse events among persons with TB using in-person vs. electronic directly observed therapy
- PMID: 37880884
- PMCID: PMC10794055
- DOI: 10.5588/ijtld.22.0594
Adverse events among persons with TB using in-person vs. electronic directly observed therapy
Abstract
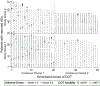
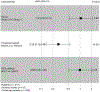
BACKGROUND: We evaluated patient safety within a randomized crossover trial comparing electronic directly observed therapy (eDOT) to in-person DOT (ipDOT) in persons undergoing TB treatment in New York City, NY, USA.METHODS: Participant symptoms, symptom severity, and clinical management were documented. We assessed adverse event reports (AERs) by DOT method during the two-period crossover. Using Cox proportional-hazards mixed-effects models, we estimated the adjusted hazard ratio (aHR) of participants reporting an adverse event (AE) vs. not reporting an AE.RESULTS: Of 211 participants, 57 (27.0%) reported AEs during the two-period crossover; of these, 54.4% (31/57) were reported while using eDOT vs. 45.6% (26/57) while using ipDOT. Controlling for study group and period, the aHR for eDOT vs. ipDOT was 0.98 (95% CI 0.49-1.93). Although statistically not significant, the wide confidence intervals suggest that a significant association cannot be entirely ruled out. Gastrointestinal symptoms were most frequently reported (42.1%, 24/57). AER types and severity did not differ significantly by DOT method. Days from symptom onset to medical attention was similar across DOT methods (median: 1.0 day, IQR 0.0-2.0). No participants switched DOT methods due to AERs or monitoring concerns.CONCLUSION: Further evaluation to ascertain whether AERs differ when patients use eDOT vs. ipDOT is warranted.
CONTEXTE :: Nous avons évalué la sécurité des patients dans le cadre d’un essai croisé randomisé comparant la thérapie électronique sous observation directe (eDOT) à la thérapie en personne (ipDOT) chez des personnes suivant un traitement contre la TB dans la ville de New York, NY, États-Unis.
MÉTHODES :: Les symptôms des participants, leur gravité et la prise en charge clinique ont été documentés. Nous avons évalué les rapports d’événements indésirables (AERs) en fonction de la méthode DOT pendant la période croisée de 2 ans. À l’aide de modèles d’effets mixtes à hasards proportionnels de Cox, nous avons estimé le rapport de risque ajusté (aHR) entre les participants ayant signalé un événement indésirable (AE) et ceux n’ayant pas signalé d’AE.
RÉSULTATS :: Sur 211 participants, 57 (27,0%) ont signalé des AE au cours de la période croisée de 2 ans; parmi ceux-ci, 54,4% (31/57) ont été signalés lors de l’utilisation de l’eDOT contre 45,6% (26/57) lors de l’utilisation de l’ipDOT. En tenant compte du groupe d’ étude et de la période, le aHR pour l’eDOT par rapport à l’ipDOT était de 0,98 (IC 95% 0,49–1,93). Bien que statistiquement non significatif, les larges intervalles de confiance suggérent qu’une association significative ne peut pas être totalement exclue. Les symptômes gastro-intestinaux ont été les plus frequémment signalés (24/57; 42,1%). Les types de AER et leur gravité ne differaiént pas significativement en fonction de la méthode DOT. Le délai entre l’apparition des symptômes et l’obtention d’un avis médical était similaire pour toutes les méthodes DOT (médiane : 1,0 jour; intervalle interquartile 0,0–2,0). Aucun participant n’a changé de méthode DOT enraison d’effets indésirables ou de problèmes de surveillance.
CONCLUSION :: Une évaluation plus poussée est justifiée pour déterminer si les AERs diffèrent lorsque les patients utilisent l’eDOT par rapport à l’ipDOT.
Figures
References
-
- World Health Organization. Global diffusion of eHealth: making universal health coverage achievable. Report of the third global survey on eHealth. Geneva, Switzerland: WHO, 2016.
-
- World Health Organization. WHO guideline recommendations on digital interventions for health system strengthening. Geneva, Switzerland: WHO, 2019. - PubMed
-
- Roundtable on the Promotion of Health Equity and the Elimination of Health Disparities; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. The promises and perils of digital strategies in achieving health equity: workshop summary. Washington, DC, USA: National Academies Press, 2016. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

