Access and use of WHO essential medicines in Italy
- PMID: 37881343
- PMCID: PMC10595003
- DOI: 10.3389/fpubh.2023.1211208
Access and use of WHO essential medicines in Italy
Abstract
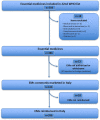
Background: Many countries use the WHO Essential Medicines List (EML) as a guide for health policy choices to promote the efficient use of healthcare resources or adopt the concept of essential medicines (EMs) to develop their own national list of essential medicines. The aim of this study is to analyse the availability and use of medicines included in the 22nd WHO EML in Italy.
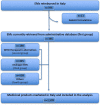
Methods: Using the ATC code (5th level), a comparison was made between the medicines included in the WHO EML and those retrieved from the Italian Medicines Agency (AIFA) database. The availability (regulatory and reimbursement status) of EMs, as well as the market share in expenditure (million euros) and consumption [measured in WHO-defined daily doses (DDDs)], compared to all reimbursed medicines in 2021, were analysed.
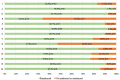
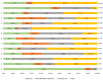
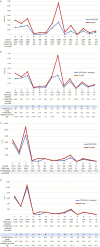
Results: In 2021, approximately 85.2% (n = 414) of medicines included in the WHO EML were commonly marketed in Italy. Of these, 396 EMs were fully reimbursed by the Italian National Healthcare Service (INHS), corresponding to 81.5% (396/486) of the WHO EML, while the remaining 18.5% (90/486) were neither authorised (n = 72) nor reimbursed (n = 18). The study found a low coverage for anti-parasitic, insecticides, and repellent products (ATC P) in addition to medicines for the genitourinary system and sex hormones (ATC G). Even though medicines on the WHO EML, including therapeutic alternatives, accounted for ~48.5% of the expenditure for medicines reimbursed by INHS, the list covered 74% of all national drug consumed. Novel high-cost therapies indicated in high-prevalence diseases and rare conditions, mostly antineoplastic and immune-modulating agents (ATC L) not included in the WHO EML, were also guaranteed.
Conclusions: In Italy, high coverage of EMs was found. It was largely reimbursed by the INHS, even when compared to other European countries. Essential medicines represented a high percentage of the overall expenditure and consumption in Italy. The WHO EML could be an important tool to guide the health policy choices of high-income countries, although a more frequent update and easier access to information on rejected medicines are needed.
Keywords: drug consumption; essential medicines; expenditure; health policy; national formulary.
Copyright © 2023 Petrella, Fortinguerra, Cangini, Pierantozzi and Trotta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO Expert Committee on the Selection of Essential Drugs World Health Organization. (1977). The Selection of Essential Drugs: Report of a WHO Expert Committee [Meeting Held in Geneva from 17 to 21 October 1977]. World Health Organization. Available online at: https://apps.who.int/iris/handle/10665/41272 (accessed September 7, 2023).
-
- World Health Organization. Promoting Rational Use of Medicines: Core Components. (2002). World Health Organization. Available online at: https://apps.who.int/iris/handle/10665/67438 (accessed September 7, 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

