Dehydroabietane-type bifunctional organocatalysts in asymmetric synthesis: recent progress
- PMID: 37881754
- PMCID: PMC10594059
- DOI: 10.1039/d3ra06715g
Dehydroabietane-type bifunctional organocatalysts in asymmetric synthesis: recent progress
Abstract
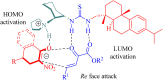
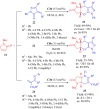
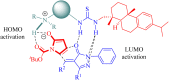
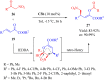
Dehydroabietane-type bifunctional organocatalysts derived from rosane-type diterpenes of dehydroabietic acid (DHAA) and dehydroabietylamine (DA) have been utilized in a wide variety of highly enantioselective reactions. Since one well-documented review exclusively reported on the development of terpene-derived bifunctional thioureas in asymmetric organocatalysis in 2013, fragmentary progress on the dehydroabietane-type bifunctional thioureas and squaramides has been mentioned in other reviews. In this mini-review, we systematically analyze and reorganize the published literature on dehydroabietane-type bifunctional organocatalysts in the recent decade according to the type of catalysts. Our aim is for this review to provide helpful research information and serve as a foundation for further design and application of rosin-based organocatalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

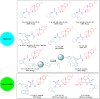
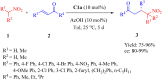


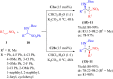

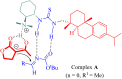


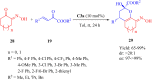
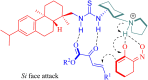
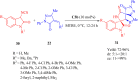
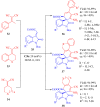

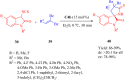

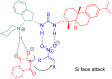

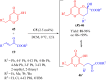

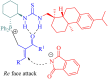
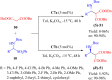
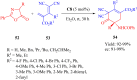
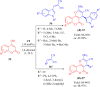

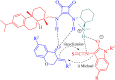

References
-
- Silvestre A. J. D. and Gandini A., Monomers, Polymers and Composites from Renewable Resources, M. N. Belgacem and A. Gandini, Elsevier, Amsterdam, The Netherlands, 2008, ch. 4, pp. 67–88
-
- Yadav B. K. Gidwani B. Vyas A. J. Bioact. Compat. Polym. 2016;31:111–126.
-
- Faustino C. Neto Í. Fonte P. Macedo A. Curr. Pharm. Design. 2018;24:4362–4375. - PubMed
-
- Wiemann J. Al-Harrasi A. Csuk R. Anti-Cancer Agents Med. Chem. 2020;20:1756–1767. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

