Cerebral venous sinus thrombosis and thrombocytopenia due to heparin-independent anti-PF4 antibodies after adenovirus infection
- PMID: 37881869
- PMCID: PMC11141643
- DOI: 10.3324/haematol.2023.284127
Cerebral venous sinus thrombosis and thrombocytopenia due to heparin-independent anti-PF4 antibodies after adenovirus infection
Figures
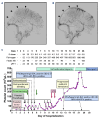

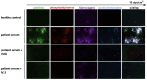
Comment in
-
Adenovirus-associated thrombosis and thrombocytopenia: an emerging anti-PF4 disorder.Haematologica. 2024 Jun 1;109(6):1651-1652. doi: 10.3324/haematol.2023.284460. Haematologica. 2024. PMID: 38328867 Free PMC article. No abstract available.
Comment on
-
Adenovirus-Associated Thrombocytopenia, Thrombosis, and VITT-like Antibodies.N Engl J Med. 2023 Aug 10;389(6):574-577. doi: 10.1056/NEJMc2307721. N Engl J Med. 2023. PMID: 37590457 No abstract available.
References
-
- Ropper AH, Klein JP. Cerebral venous thrombosis. N Engl J Med. 2021;385(1):59-64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

