Diabetes mellitus promotes the nasal colonization of high virulent Staphylococcus aureus through the regulation of SaeRS two-component system
- PMID: 37882148
- PMCID: PMC10796126
- DOI: 10.1080/22221751.2023.2276335
Diabetes mellitus promotes the nasal colonization of high virulent Staphylococcus aureus through the regulation of SaeRS two-component system
Abstract
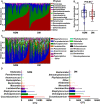
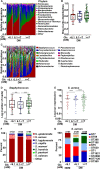
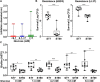
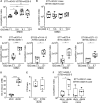
Diabetic foot infections are a common complication of diabetes. Staphylococcus aureus is frequently isolated from diabetic foot infections and commonly colonizes human nares. According to the study, the nasal microbiome analysis revealed that diabetic patients had a significantly altered nasal microbial composition and diversity. Typically, the fasting blood glucose (FBG) level had an impact on the abundance and sequence type (ST) of S. aureus in diabetic patients. We observed that highly virulent S. aureus ST7 strains were more frequently colonized in diabetic patients, especially those with poorly controlled FBG, while ST59 was dominant in healthy individuals. S. aureus ST7 strains were more resistant to human antimicrobial peptides and formed stronger biofilms than ST59 strains. Critically, S. aureus ST7 strains displayed higher virulence compared to ST59 strains in vivo. The dominance of S. aureus ST7 strains in hyperglycemic environment is due to the higher activity of the SaeRS two-component system (TCS). S. aureus ST7 strains outcompeted ST59 both in vitro, and in nasal colonization model in diabetic mice, which was abolished by the deletion of the SaeRS TCS. Our data indicated that highly virulent S. aureus strains preferentially colonize diabetic patients with poorly controlled FBG through SaeRS TCS. Detection of S. aureus colonization and elimination of colonizing S. aureus are critical in the care of diabetic patients with high FBG.
Keywords: Diabetes; SaeRS two-component system; Staphylococcus aureus; colonization; microbiome.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Virulence adaption to environment promotes the age-dependent nasal colonization of Staphylococcus aureus.Emerg Microbes Infect. 2022 Dec;11(1):1402-1415. doi: 10.1080/22221751.2022.2074316. Emerg Microbes Infect. 2022. PMID: 35508433 Free PMC article.
-
Virulent Staphylococcus aureus colonizes pediatric nares by resisting killing of human antimicrobial peptides.Int J Med Microbiol. 2022 Feb;312(2):151550. doi: 10.1016/j.ijmm.2022.151550. Epub 2022 Jan 24. Int J Med Microbiol. 2022. PMID: 35091347
-
Methicillin-resistant Staphylococcus aureus nasal carriage and infection among patients with diabetic foot ulcer.J Microbiol Immunol Infect. 2020 Apr;53(2):292-299. doi: 10.1016/j.jmii.2018.03.005. Epub 2018 Jun 4. J Microbiol Immunol Infect. 2020. PMID: 29907536
-
Pre-surgical Nasal Decolonization of Staphylococcus aureus: A Health Technology Assessment.Ont Health Technol Assess Ser. 2022 Aug 23;22(4):1-165. eCollection 2022. Ont Health Technol Assess Ser. 2022. PMID: 36160757 Free PMC article. Review.
-
How does Staphylococcus aureus successfully colonize the nasal cavity.Crit Rev Microbiol. 2025 Apr 17:1-20. doi: 10.1080/1040841X.2025.2486171. Online ahead of print. Crit Rev Microbiol. 2025. PMID: 40243667 Review.
References
-
- Saeedi P, Petersohn I, Salpea P, et al. . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. 2019 Nov;157:107843, doi:10.1016/j.diabres.2019.107843 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
