PGAM5 degrades PDCoV N protein and activates type I interferon to antagonize viral replication
- PMID: 37882521
- PMCID: PMC10688367
- DOI: 10.1128/jvi.01470-23
PGAM5 degrades PDCoV N protein and activates type I interferon to antagonize viral replication
Abstract
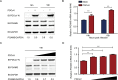
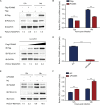
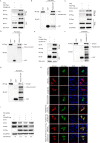
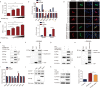
As a member of the δ-coronavirus family, porcine deltacoronavirus (PDCoV) is a vital reason for diarrhea in piglets, which can contribute to high morbidity and mortality rates. Initially identified in Hong Kong in 2012, the virus has rapidly spread worldwide. During PDCoV infection, the virus employs evasion mechanisms to evade host surveillance, while the host mounts corresponding responses to impede viral replication. Our research has revealed that PDCoV infection down-regulates the expression of PGAM5 to promote virus replication. In contrast, PGAM5 degrades PDCoV N through autophagy by interacting with the cargo receptor P62 and the E3 ubiquitination ligase STUB1. Additionally, PGAM5 interacts with MyD88 and TRAF3 to activate the IFN signal pathway, resulting in the inhibition of viral replication.
Keywords: N protein; PDCoV; PGAM5; autophagy; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 86:3995–4008. doi:10.1128/JVI.06540-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- no.2021YFD1801102/MOST | National Key Research and Development Program of China (NKPs)
- no.32272999 and 32102665/MOST | National Natural Science Foundation of China (NSFC)
- no.Y2022QC28/MOA | CAFS | Central Public-interest Scientific Institution Basal Research Fund, Chinese Academy of Fishery Sciences (Central Public-interest Scientific Institution Basal Research Fund, CAFS)
LinkOut - more resources
Full Text Sources
Research Materials

