Biofilm formation and cell plasticity drive diazotrophy in an anoxygenic phototrophic bacterium
- PMID: 37882569
- PMCID: PMC10686084
- DOI: 10.1128/aem.01027-23
Biofilm formation and cell plasticity drive diazotrophy in an anoxygenic phototrophic bacterium
Abstract
The contribution of non-cyanobacterial diazotrophs (NCDs) to total N2 fixation in the marine water column is unknown, but their importance is likely constrained by the limited availability of dissolved organic matter and low O2 conditions. Light could support N2 fixation and growth by NCDs, yet no examples from bacterioplankton exist. In this study, we show that the phototrophic NCD, Rhodopseudomonas sp. BAL398, which is a member of the diazotrophic community in the surface waters of the Baltic Sea, can utilize light. Our study highlights the significance of biofilm formation for utilizing light and fixing N2 under oxic conditions and the role of cell plasticity in regulating these processes. Our findings have implications for the general understanding of the ecology and importance of NCDs in marine waters.
Keywords: Baltic Sea; N2 fixation; biofilm; light; nutrients; oxygen (O2); purple non-sulfur bacteria (PNSB); water column.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


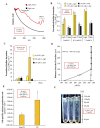

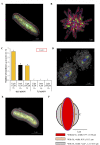
References
-
- Riemann L, Farnelid H, Steward GF. 2010. Nitrogenase genes in non-cyanobacterial plankton: prevalence, diversity and regulation in marine waters. Aquat Microb Ecol. 61:235–247. doi:10.3354/ame01431 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

