The Role of In Situ/ Operando IR Spectroscopy in Unraveling Adsorbate-Induced Structural Changes in Heterogeneous Catalysis
- PMID: 37882638
- PMCID: PMC10636737
- DOI: 10.1021/acs.chemrev.3c00372
The Role of In Situ/ Operando IR Spectroscopy in Unraveling Adsorbate-Induced Structural Changes in Heterogeneous Catalysis
Abstract
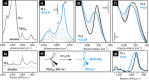
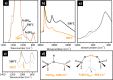
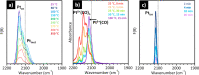
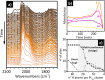
Heterogeneous catalysts undergo thermal- and/or adsorbate-induced dynamic changes under reaction conditions, which consequently modify their catalytic behavior. Hence, it is increasingly crucial to characterize the properties of a catalyst under reaction conditions through the so-called "operando" approach. Operando IR spectroscopy is probably one of the most ubiquitous and versatile characterization methods in the field of heterogeneous catalysis, but its potential in identifying adsorbate- and thermal-induced phenomena is often overlooked in favor of other less accessible methods, such as XAS spectroscopy and high-resolution microscopy. Without detracting from these techniques, and while aware of the enormous value of a multitechnique approach, the purpose of this Review is to show that IR spectroscopy alone can provide relevant information in this field. This is done by discussing a few selected case studies from our own research experience, which belong to the categories of both "single-site"- and nanoparticle-based catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

















References
-
- Yang A. C.; Garland C. W. Infrared Studies of Carbon Monoxide Chemisorbed on Rhodium. J. Phys. Chem. 1957, 61, 1504–1512. 10.1021/j150557a013. - DOI
Publication types
LinkOut - more resources
Full Text Sources

