Treatment-Resistant Depression in Real-World Clinical Practice: A Systematic Literature Review of Data from 2012 to 2022
- PMID: 37882883
- PMCID: PMC10796703
- DOI: 10.1007/s12325-023-02700-0
Treatment-Resistant Depression in Real-World Clinical Practice: A Systematic Literature Review of Data from 2012 to 2022
Abstract
Objective: Real-world evidence in treatment-resistant depression (TRD; commonly defined as non-response to ≥ 2 consecutive treatments at adequate dosage and duration) is lacking. A systematic literature review was conducted to understand disease burden and treatment outcomes for patients with TRD, studied in a real-world setting over the last decade.
Data sources: A literature search was conducted in May 2022 in MEDLINE, Embase, The Cochrane Libraries and PsycINFO, comprising studies published from 2012 to 2022. Bibliographies of all relevant identified systematic reviews and relevant conference proceedings from 2020 to 2022 were manually hand-searched.
Study selection: Real-world studies, including cohort, cross-sectional, case-control, chart review and registry studies, published in English and reporting outcomes in adults with TRD, were included.
Data extraction: Extracted data included study and baseline disease characteristics, treatment type, treatment response, clinical outcomes and health-related quality of life.
Results: Twenty studies were included. Criteria for TRD varied, but patients typically experienced long-lasting depression (range 1.4 to 16.5 years). Across studies, mean disease severity scores demonstrated moderate to severe depression, reflecting a high burden of disease at baseline. Remission rates were typically low but generally increased with longer follow-up durations. However, the heterogeneity of interventions, follow-up durations (range 2 weeks to 9.4 years) and assessment tools precluded their quantitative synthesis. Studies were frequently limited by low sample size (range 14 to 411 patients) and health-related quality of life was infrequently assessed.
Conclusions: There is a lack of clinical consensus regarding the definition, assessment and monitoring of TRD in real-world practice. Nevertheless, TRD carries a high burden of illness and there is an unmet need for faster and more effective treatments. To better understand the personal burden of affected patients, future studies would benefit from standardisation of severity assessment and measures of treatment effectiveness, as well as greater consideration of health-related quality of life.
Keywords: Major depressive disorder; Real-world evidence; Systematic literature review; Treatment-resistant depression.
Plain language summary
Many people continue to experience depression even after trying two or more medications. This is called treatment-resistant depression (TRD). Most of the information we have on TRD comes from clinical trials, which take place under tightly-controlled conditions. It is important to understand the effects of TRD and TRD treatments on people in their day-to-day lives. Researchers studying people’s day-to-day lives call this researching in a “real-world setting”. We searched for studies carried out in real-world settings in the last 10 years. We found 20 relevant studies. As these studies were in real-world settings, there were many differences between them, including differences in how TRD was diagnosed, the treatments used, how long people were monitored and how results were measured. This made it difficult to compare how successful different treatments were. Most studies included a small number of people and monitored them for a relatively short time. We found people with TRD had usually lived with it for many years and their symptoms were moderate or severe. Only two studies asked people how TRD affected their lives. These two studies found health-related quality of life and work productivity was low. Most studies found lots of people still had symptoms of depression after treatment. However, symptoms typically improved more when studies monitored people for a longer time. To improve our knowledge of TRD, future studies should monitor more people for longer and use the same ways of measuring results. They should also ask how TRD affects people’s daily lives.
© 2023. The Author(s).
Conflict of interest statement
Albino J. Oliveira-Maia: Received grants from Schuhfried GmBH, Janssen and Compass Pathways, Ltd; received payment, honoraria, travel support or advisory board fees from MSD, Janssen, Angelini, Neurolite AG, and the European Monitoring Centre for Drugs and Drug Addiction; investigator-driven research funded by Fundação para Ciência e Tecnologia (PTDC/SAU-NUT/3507/2021; PTDC/MED-NEU/1552/2021; PTDC/MED-NEU/31331/2017), Fundação para Ciência e Tecnologia and FEDER (PTDC/MED-NEU/30845/2017_LISBOA-01–0145-FEDER-030845; FCT-PTDC/MEC-PSQ/30302/2017-IC&DTLISBOA-01-0145-FEDER), the European Commission Horizon 2020 program (H2020-SC1-2017-CNECT-2-777167-ΒΟUNCE; H2020-SC1-DTH-2019-875358-FAITH), the European Research Council (grant ERC-2020-STG-Grant agreement 950357) and the European Joint Programme in Rare Diseases (Joint Translational Call 2019) through Fundação para Ciência e Tecnologia (EJPRD/0001/2020); Vice-President of the Portuguese Society for Psychiatry and Mental Health; Head of the Psychiatry Working Group for the National Board of Medical Examination (GPNA) at the Portuguese Medical Association and Portuguese Ministry of Health. Eric Constant: Received honoraria, advisory board fees and travel support from AstraZeneca, BMS, Lilly, Wyeth, Servier, Janssen Cilag, Pfizer, Lundbeck, Viatris and Teva. Tetsuro Ito, Yerkebulan Kambarov, Siobhán Mulhern-Haughey, Christian von Holt: Employees of Janssen; hold Johnson & Johnson company stocks/stock options. Ania Bobrowska, Hannah Luedke: Employees of Costello Medical, UK.
Figures
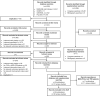
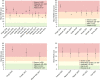
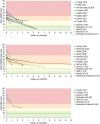
References
-
- National Institute for Health and Care Excellence. Depression in adults: treatment and management (NG222) 2022. https://www.nice.org.uk/guidance/ng222/resources/depression-in-adults-tr.... Accessed 7 Dec 2022. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

