Gasdermin C sensitizes tumor cells to PARP inhibitor therapy in cancer models
- PMID: 37883181
- PMCID: PMC10760963
- DOI: 10.1172/JCI166841
Gasdermin C sensitizes tumor cells to PARP inhibitor therapy in cancer models
Abstract
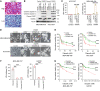
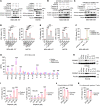
Several poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) are approved by FDA to treat cancer with BRCA mutations. BRCA mutations are considered to fuel a PARPi killing effect by inducing apoptosis. However, resistance to PARPi is frequently observed in the clinic due to an incomplete understanding on the molecular basis of PARPi function and a lack of good markers, beyond BRCA mutations, to predict response. Here, we show that gasdermin C (GSDMC) sensitized tumor cells to PARPi in vitro and in immunocompetent mice and caused durable tumor regression in an immune-dependent manner. A high expression level of GSDMC predicted better response to PARPi treatment in patients with triple-negative breast cancer (TNBC). PARPi treatment triggered GSDMC/caspase-8-mediated cancer cell pyroptosis (CCP) that enhanced PARPi killing of tumor cells. GSDMC-mediated CCP increased memory CD8+ T cell population in lymph node (LN), spleen, and tumor and, thus, promoted cytotoxic CD8+ T cell infiltration in the tumor microenvironment. T cell-derived granzyme B (GZMB) activated caspase-6, which subsequently cleaved GSDMC to induce pyroptosis. Interestingly, IFN-γ induced GSDMC expression, which, in turn, enhanced the cytotoxicity of PARPi and T cells. Importantly, GSDMC promoted tumor clearance independent of BRCA deficiency in multiple cancer types with PARPi treatment. This study identifies a general marker and target for PARPi therapy and offers insights into the mechanism of PARPi function.
Keywords: Cancer; Drug therapy; Oncology; T cells; Therapeutics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

