CD22L Conjugation to Insulin Attenuates Insulin-Specific B Cell Activation
- PMID: 37883211
- PMCID: PMC11034786
- DOI: 10.1021/acs.bioconjchem.3c00391
CD22L Conjugation to Insulin Attenuates Insulin-Specific B Cell Activation
Abstract
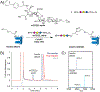
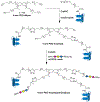
Pancreatic islet-reactive B lymphocytes promote Type 1 diabetes (T1D) by presenting an antigen to islet-destructive T cells. Teplizumab, an anti-CD3 monoclonal, delays T1D onset in patients at risk, but additional therapies are needed to prevent the disease entirely. Therefore, bifunctional molecules were designed to selectively inhibit T1D-promoting anti-insulin B cells by conjugating a ligand for the B cell inhibitory receptor CD22 (i.e., CD22L) to insulin, which permit these molecules to concomitantly bind to anti-insulin B cell receptors (BCRs) and CD22. Two prototypes were synthesized: 2:2 insulin-CD22L conjugate on a 4-arm PEG backbone, and 1:1 insulin-CD22L direct conjugate. Transgenic mice (125TgSD) expressing anti-insulin BCRs provided cells for in vitro testing. Cells were cultured with constructs for 3 days, then assessed by flow cytometry. Duplicate wells with anti-CD40 simulated T cell help. A 2-insulin 4-arm PEG control caused robust proliferation and activation-induced CD86 upregulation. Anti-CD40 further boosted these effects. This may indicate that BCR-cross-linking occurs when antigens are tethered by the PEG backbone as soluble insulin alone has no effect. Addition of CD22L via the 2:2 insulin-CD22L conjugate restored B cell properties to that of controls without an additional beneficial effect. In contrast, the 1:1 insulin-CD22L direct conjugate significantly reduced anti-insulin B cell proliferation in the presence of anti-CD40. CD22L alone had no effect, and the constructs did not affect the WT B cells. Thus, multivalent antigen constructs tend to activate anti-insulin B cells, while monomeric antigen-CD22L conjugates reduce B cell activation in response to simulated T cell help and reduce pathogenic B cell numbers without harming normal cells. Therefore, monomeric antigen-CD22L conjugates warrant futher study and may be promising candidates for preclinical trials to prevent T1D without inducing immunodeficiency.
Conflict of interest statement
Notes
The authors declare the following competing financial interest(s): KDA, MPF, and CJB are co-inventors on a patent application related to this work filed by the University of Kansas on conjugates with inhibitory receptor ligands to limit the activation of insulin-binding B cells (WO2022165016A1).
Figures
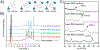




References
-
- Shlomchik MJ Sites and Stages of Autoreactive B Cell Activation and Regulation. Immunity 2008, 28 (1), 18–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

