PRMT5 mediates FoxO1 methylation and subcellular localization to regulate lipophagy in myogenic progenitors
- PMID: 37883229
- PMCID: PMC10727913
- DOI: 10.1016/j.celrep.2023.113329
PRMT5 mediates FoxO1 methylation and subcellular localization to regulate lipophagy in myogenic progenitors
Abstract
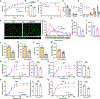
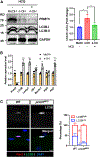
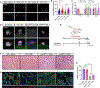
Development is regulated by various factors, including protein methylation status. While PRMT5 is well known for its roles in oncogenesis by mediating symmetric di-methylation of arginine, its role in normal development remains elusive. Using Myod1Cre to drive Prmt5 knockout in embryonic myoblasts (Prmt5MKO), we dissected the role of PRMT5 in myogenesis. The Prmt5MKO mice are born normally but exhibit progressive muscle atrophy and premature death. Prmt5MKO inhibits proliferation and promotes premature differentiation of embryonic myoblasts, reducing the number and regenerative function of satellite cells in postnatal mice. Mechanistically, PRMT5 methylates and destabilizes FoxO1. Prmt5MKO increases the total FoxO1 level and promotes its cytoplasmic accumulation, leading to activation of autophagy and depletion of lipid droplets (LDs). Systemic inhibition of autophagy in Prmt5MKO mice restores LDs in myoblasts and moderately improves muscle regeneration. Together, PRMT5 is essential for muscle development and regeneration at least partially through mediating FoxO1 methylation and LD turnover.
Keywords: CP: Developmental biology; CP: Molecular biology; PRMT; PTM; SCs; autophagy; myogenesis; posttranslational modification; protein arginine methyltransferase; satellite cells.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Pallafacchina G, Blaauw B, and Schiaffino S (2013). Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr. Metabol. Cardiovasc. Dis. 23, S12–S18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

