Eflornithine as Postimmunotherapy Maintenance in High-Risk Neuroblastoma: Externally Controlled, Propensity Score-Matched Survival Outcome Comparisons
- PMID: 37883734
- PMCID: PMC10730038
- DOI: 10.1200/JCO.22.02875
Eflornithine as Postimmunotherapy Maintenance in High-Risk Neuroblastoma: Externally Controlled, Propensity Score-Matched Survival Outcome Comparisons
Abstract
Purpose: Long-term survival in high-risk neuroblastoma (HRNB) is approximately 50%, with mortality primarily driven by relapse. Eflornithine (DFMO) to reduce risk of relapse after completion of immunotherapy was investigated previously in a single-arm, phase II study (NMTRC003B; ClinicalTrials.gov identifier: NCT02395666) that suggested improved event-free survival (EFS) and overall survival (OS) compared with historical rates in a phase III trial (Children Oncology Group ANBL0032; ClinicalTrials.gov identifier: NCT00026312). Using patient-level data from ANBL0032 as an external control, we present new analyses to further evaluate DFMO as HRNB postimmunotherapy maintenance.
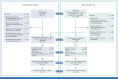
Patients and methods: NMTRC003B (2012-2016) enrolled patients with HRNB (N = 141) after standard up-front or refractory/relapse treatment who received up to 2 years of continuous treatment with oral DFMO (750 ± 250 mg/m2 twice a day). ANBL0032 (2001-2015) enrolled patients with HRNB postconsolidation, 1,328 of whom were assigned to dinutuximab (ch.14.18) treatment. Selection rules identified 92 NMTRC003B patients who participated in (n = 87) or received up-front treatment consistent with (n = 5) ANBL0032 (the DFMO/treated group) and 852 patients from ANBL0032 who could have been eligible for NMTRC003B after immunotherapy, but did not enroll (the NO-DFMO/control group). The median follow-up time for DFMO/treated patients was 6.1 years (IQR, 5.2-7.2) versus 5.0 years (IQR, 3.5-7.0) for NO-DFMO/control patients. Kaplan-Meier and Cox regression compared EFS and OS for overall groups, 3:1 (NO-DFMO:DFMO) propensity score-matched cohorts balanced on 11 baseline demographic and disease characteristics with exact matching on MYCN, and additional sensitivity analyses.
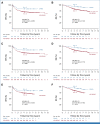
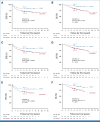
Results: DFMO after completion of immunotherapy was associated with improved EFS (hazard ratio [HR], 0.50 [95% CI, 0.29 to 0.84]; P = .008) and OS (HR, 0.38 [95% CI, 0.19 to 0.76]; P = .007). The results were confirmed with propensity score-matched cohorts and sensitivity analyses.
Conclusion: The externally controlled analyses presented show a relapse risk reduction in patients with HRNB treated with postimmunotherapy DFMO.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Park JR, Eggert A, Caron H: Neuroblastoma: Biology, prognosis, and treatment. Hematol Oncol Clin North Am 24:65-86, 2010 - PubMed

