Anti-PF4 immunothrombosis without proximate heparin or adenovirus vector vaccine exposure
- PMID: 37883798
- PMCID: PMC10862238
- DOI: 10.1182/blood.2023022136
Anti-PF4 immunothrombosis without proximate heparin or adenovirus vector vaccine exposure
Abstract
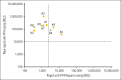
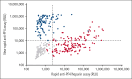
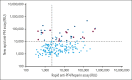
Platelet-activating anti-platelet factor 4 (PF4)/heparin antibodies and anti-PF4 antibodies cause heparin-induced thrombocytopenia (HIT) and vaccine-induced immune thrombocytopenia and thrombosis (VITT), respectively. Diagnostic and treatment considerations differ somewhat between HIT and VITT. We identified patients with thrombocytopenia and thrombosis without proximate heparin exposure or adenovirus-based vaccination who tested strongly positive by PF4/polyanion enzyme-immunoassays and negative/weakly positive by heparin-induced platelet activation (HIPA) test but strongly positive by PF4-induced platelet activation (PIPA) test (ie, VITT-like profile). We tested these patients by a standard chemiluminescence assay that detects anti-PF4/heparin antibodies found in HIT (HemosIL AcuStar HIT-IgG(PF4-H)) as well as a novel chemiluminescence assay for anti-PF4 antibodies found in VITT. Representative control sera included an exploratory anti-PF4 antibody-positive but HIPA-negative/weak cohort obtained before 2020 (n = 188). We identified 9 patients with a clinical-pathological profile of a VITT-like disorder in the absence of proximate heparin or vaccination, with a high frequency of stroke (arterial, n = 3; cerebral venous sinus thrombosis, n = 4), thrombocytopenia (median platelet count nadir, 49 × 109/L), and hypercoagulability (greatly elevated D-dimer levels). VITT-like serological features included strong reactivity by PIPA (aggregation <10 minutes in 9/9 sera) and positive testing in the novel anti-PF4 chemiluminescence assay (3/9 also tested positive in the anti-PF4/heparin chemiluminescence assay). Our exploratory cohort identified 13 additional patient sera obtained before 2020 with VITT-like anti-PF4 antibodies. Platelet-activating VITT-like anti-PF4 antibodies should be considered in patients with thrombocytopenia, thrombosis, and very high D-dimer levels, even without a proximate exposure to heparin or adenovirus vector vaccines.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.G. reports grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme (MSD), Bristol Myers Squibb (BMS), Paringenix, Bayer HealthCare, Gore Inc, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, and Werfen; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V. outside the submitted work. O.E., P.D., M.B., S.R.P., R.T., J.S., R.L., and M.P. are employees of Werfen. O.E., P.D., M.B., R.L., and A.G. have filed a patent application (PCT/EP2023/062615) covering the design and use of the new rapid anti-PF4 assay. E.L.-L. has received lecture honoraria and advisory fees from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, Portola, CSL Behring, Viatris, Werfen, Norgine, and Aspen; and institutional research support from Bayer AG, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, and CSL Behring for a different research project. F.L. has received personal fees for lectures or consultancy from Alexion, AstraZeneca, Bayer, BioMarin, BioNTech, Bristol Myers Squibb, Chugai, CSL Behring, Daiichi Sankyo, Grifols, Janssen-Cilag, LEO Pharma, Mitsubishi Tanabe Pharma, Novo Nordisk, Pfizer, Roche, Swedish Orphan Biovitrum, Takeda, Viatris, and Werfen; and institutional research support from Bayer, Chugai, CSL Behring, Intersero, Novo Nordisk, Pfizer, and Swedish Orphan Biovitrum. L.A. reports institutional research support from Bayer, CSL Behring, Novartis, Novo Nordisk, Octapharma, Roche, Sobi, Takeda/Shire, and Werfen; and fees for lectures or consultancy from Bayer, Biotest, Boehringer-Ingelheim, CSL Behring, Daiichi Sankyo, Novo Nordisk, OrPha Swiss, Roche, Sanofi-Aventis, Sanofi-Genzyme, Siemens, Sobi, Takeda/Shire, Viatris, and Werfen. M.E. reports grants from Bayer; and fees paid to the Charité from Abbot, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Novartis, and Pfizer, all are outside the submitted work. T.T. reports personal fees and/or other from Bristol Myers Squibb, Pfizer, Bayer, Chugai Pharma, Novo Nordisk, Novartis, Daichii Sankyo, and LFB Pharma, all of which are outside the submitted work. L.S. receives a young investigator grant of the medical faculty of the Universitätsmedizin Greifswald and a Global Research Award of the American Society of Hematology. T.H. has received lecture honoraria and advisory fees from CSL Behring, Werfen, and Takeda. C.B. has received lecture honoraria from AbbVie; and travel grants from Pfizer and Sanofi outside the submitted work. T.E.W. has received lecture honoraria from Werfen (Instrumentation Laboratory), and royalties from Informa (Taylor & Francis); consulting service fees from Ergomed, Paradigm Pharmaceuticals, Octapharma, and Veralox Therapeutics; research funding from Werfen (Instrumentation Laboratory); and has provided expert witness testimony relating to heparin-induced thrombocytopenia (HIT) and non-HIT thrombocytopenic and coagulopathic disorders. The remaining authors declare no competing financial interests.
Figures

Comment in
-
VITT-like disorder HITs the headlines.Blood. 2023 Dec 28;142(26):2229-2230. doi: 10.1182/blood.2023022900. Blood. 2023. PMID: 38153773 No abstract available.
References
-
- Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(19):1883–1884. - PubMed
-
- Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. - PubMed
-
- Warkentin T, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135(7):502–506. - PubMed
-
- Warkentin TE, Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40–51. - PubMed
-
- Greinacher A, Holtfreter B, Krauel K, et al. Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood. 2011;118(5):1395–1401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

