An autosomal-dominant childhood-onset disorder associated with pathogenic variants in VCP
- PMID: 37883978
- PMCID: PMC10645565
- DOI: 10.1016/j.ajhg.2023.10.007
An autosomal-dominant childhood-onset disorder associated with pathogenic variants in VCP
Abstract
Valosin-containing protein (VCP) is an AAA+ ATPase that plays critical roles in multiple ubiquitin-dependent cellular processes. Dominant pathogenic variants in VCP are associated with adult-onset multisystem proteinopathy (MSP), which manifests as myopathy, bone disease, dementia, and/or motor neuron disease. Through GeneMatcher, we identified 13 unrelated individuals who harbor heterozygous VCP variants (12 de novo and 1 inherited) associated with a childhood-onset disorder characterized by developmental delay, intellectual disability, hypotonia, and macrocephaly. Trio exome sequencing or a multigene panel identified nine missense variants, two in-frame deletions, one frameshift, and one splicing variant. We performed in vitro functional studies and in silico modeling to investigate the impact of these variants on protein function. In contrast to MSP variants, most missense variants had decreased ATPase activity, and one caused hyperactivation. Other variants were predicted to cause haploinsufficiency, suggesting a loss-of-function mechanism. This cohort expands the spectrum of VCP-related disease to include neurodevelopmental disease presenting in childhood.
Copyright © 2023 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
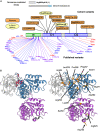


References
-
- Tresse E., Salomons F.A., Vesa J., Bott L.C., Kimonis V., Yao T.-P., Dantuma N.P., Taylor J.P. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–227. doi: 10.4161/auto.6.2.11014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

