Prospective, multi-site study of healthcare utilization after actionable monogenic findings from clinical sequencing
- PMID: 37883979
- PMCID: PMC10645563
- DOI: 10.1016/j.ajhg.2023.10.006
Prospective, multi-site study of healthcare utilization after actionable monogenic findings from clinical sequencing
Abstract
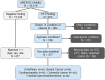
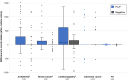
As large-scale genomic screening becomes increasingly prevalent, understanding the influence of actionable results on healthcare utilization is key to estimating the potential long-term clinical impact. The eMERGE network sequenced individuals for actionable genes in multiple genetic conditions and returned results to individuals, providers, and the electronic health record. Differences in recommended health services (laboratory, imaging, and procedural testing) delivered within 12 months of return were compared among individuals with pathogenic or likely pathogenic (P/LP) findings to matched individuals with negative findings before and after return of results. Of 16,218 adults, 477 unselected individuals were found to have a monogenic risk for arrhythmia (n = 95), breast cancer (n = 96), cardiomyopathy (n = 95), colorectal cancer (n = 105), or familial hypercholesterolemia (n = 86). Individuals with P/LP results more frequently received services after return (43.8%) compared to before return (25.6%) of results and compared to individuals with negative findings (24.9%; p < 0.0001). The annual cost of qualifying healthcare services increased from an average of $162 before return to $343 after return of results among the P/LP group (p < 0.0001); differences in the negative group were non-significant. The mean difference-in-differences was $149 (p < 0.0001), which describes the increased cost within the P/LP group corrected for cost changes in the negative group. When stratified by individual conditions, significant cost differences were observed for arrhythmia, breast cancer, and cardiomyopathy. In conclusion, less than half of individuals received billed health services after monogenic return, which modestly increased healthcare costs for payors in the year following return.
Keywords: actionable genetic findings; clinical outcomes; electronic health records; healthcare costs; healthcare utilization; monogenic sequencing; return of results; translational.
Copyright © 2023 American Society of Human Genetics. All rights reserved.
Conflict of interest statement
Declaration of interests W.K.C. is on the board of directors of Prime Medicine and D.L.V. is a consultant for Illumina and has a funded project from GeneDx.
Figures

References
-
- Grzymski J.J., Elhanan G., Morales Rosado J.A., Smith E., Schlauch K.A., Read R., Rowan C., Slotnick N., Dabe S., Metcalf W.J., et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat. Med. 2020;26:1235–1239. doi: 10.1038/s41591-020-0982-5. - DOI - PubMed
-
- Daly M.B., Pilarski R., Yurgelun M.B., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Garber J.E., et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2020;18:380–391. doi: 10.6004/jnccn.2020.0017. - DOI - PubMed
-
- Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. - DOI - PMC - PubMed
-
- Landstrom A.P., Chahal A.A., Ackerman M.J., Cresci S., Milewicz D.M., Morris A.A., Sarquella-Brugada G., Semsarian C., Shah S.H., Sturm A.C., American Heart Association Data Science and Precision Medicine Committee of the Council on Genomic and Precision Medicine and Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Hypertension; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Peripheral Vascular Disease; and Stroke Council Interpreting Incidentally Identified Variants in Genes Associated With Heritable Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circ. Genom. Precis. Med. 2023;16 doi: 10.1161/HCG.0000000000000092. - DOI - PubMed
-
- Ommen S.R., Mital S., Burke M.A., Day S.M., Deswal A., Elliott P., Evanovich L.L., Hung J., Joglar J.A., Kantor P., et al. AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- U01 HG008676/HG/NHGRI NIH HHS/United States
- U01 HG008657/HG/NHGRI NIH HHS/United States
- U01 HG008672/HG/NHGRI NIH HHS/United States
- U01 HG008679/HG/NHGRI NIH HHS/United States
- U01 HG008666/HG/NHGRI NIH HHS/United States
- U54 MD007593/MD/NIMHD NIH HHS/United States
- U01 HG008680/HG/NHGRI NIH HHS/United States
- U01 HG008673/HG/NHGRI NIH HHS/United States
- U01 HG008685/HG/NHGRI NIH HHS/United States
- U01 HG006379/HG/NHGRI NIH HHS/United States
- U01 HG008664/HG/NHGRI NIH HHS/United States
- U01 HG008701/HG/NHGRI NIH HHS/United States
- U01 HG008684/HG/NHGRI NIH HHS/United States
- U01 HG011169/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

