Integrin αvβ1 facilitates ACE2-mediated entry of SARS-CoV-2
- PMID: 37884208
- PMCID: PMC10651773
- DOI: 10.1016/j.virusres.2023.199251
Integrin αvβ1 facilitates ACE2-mediated entry of SARS-CoV-2
Abstract
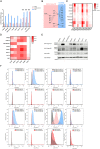
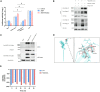
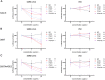
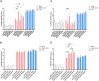
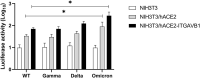
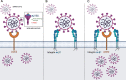
Integrins have been suggested to be involved in SARS-CoV-2 infection, but the underlying mechanisms remain largely unclear. This study aimed to investigate how integrins facilitate the ACE2-mediated cellular entry of SARS-CoV-2. We first tested the susceptibility of a panel of human cell lines to SARS-CoV-2 infection using the spike protein pseudotyped virus assay and examined the expression levels of integrins in these cell lines by qPCR, western blot and flow cytometry. We found that integrin αvβ1 was highly enriched in the SARS-CoV-2 susceptible cell lines. Additional studies demonstrated that RGD (403-405)→AAA mutant was defective in binding to integrin αvβ1 compared to its wild type counterpart, and anti-αvβ1 integrin antibodies significantly inhibited the entry of SARS-CoV-2 into the cells. Further studies using mouse NIH3T3 cells expressing human ACE2, integrin αv, integrin β1, and/or integrin αvβ1 suggest that integrin αvβ1 was unable to function as an independent receptor but could significantly facilitate the cellular entry of SASR-CoV-2. Finally, we observed that the Omicron exhibited a significant increase in the ACE2-mediated viral entry. Our findings may enhance our understanding of the pathogenesis of SARS-CoV-2 infection and offer potential therapeutic target for COVID-19.
Keywords: ACE2; COVID-19; Integrin αvβ1; SARS-CoV-2; Viral entry.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Integrin mediates cell entry of the SARS-CoV-2 virus independent of cellular receptor ACE2.J Biol Chem. 2022 Mar;298(3):101710. doi: 10.1016/j.jbc.2022.101710. Epub 2022 Feb 10. J Biol Chem. 2022. PMID: 35150743 Free PMC article.
-
In Silico, In Vitro and In Cellulo Models for Monitoring SARS-CoV-2 Spike/Human ACE2 Complex, Viral Entry and Cell Fusion.Viruses. 2021 Feb 25;13(3):365. doi: 10.3390/v13030365. Viruses. 2021. PMID: 33669132 Free PMC article.
-
SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19.Immunobiology. 2021 Jan;226(1):152021. doi: 10.1016/j.imbio.2020.152021. Epub 2020 Nov 5. Immunobiology. 2021. PMID: 33232865 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
-
Targeting the viral-entry facilitators of SARS-CoV-2 as a therapeutic strategy in COVID-19.J Med Virol. 2021 Sep;93(9):5260-5276. doi: 10.1002/jmv.27019. Epub 2021 May 3. J Med Virol. 2021. PMID: 33851732 Free PMC article. Review.
Cited by
-
Structural basis for the participation of the SARS-CoV-2 nucleocapsid protein in the template switch mechanism and genomic RNA reorganization.J Biol Chem. 2024 Nov;300(11):107834. doi: 10.1016/j.jbc.2024.107834. Epub 2024 Sep 27. J Biol Chem. 2024. PMID: 39343000 Free PMC article. Review.
-
Integrin beta 1 facilitates non-enveloped hepatitis E virus cell entry through the recycling endosome.Nat Commun. 2025 Jun 26;16(1):5403. doi: 10.1038/s41467-025-61071-y. Nat Commun. 2025. PMID: 40571699 Free PMC article.
-
Mechanisms and Research Methods of Protein Modification in Virus Entry.Appl Biochem Biotechnol. 2025 Jul 19. doi: 10.1007/s12010-025-05333-x. Online ahead of print. Appl Biochem Biotechnol. 2025. PMID: 40682621 Review.
-
Early nintedanib deployment in COVID-19 interstitial lung disease (ENDCOV-I): study protocol of a randomised, double-blind, placebo-controlled trial.BMJ Open Respir Res. 2025 Apr 10;12(1):e002323. doi: 10.1136/bmjresp-2024-002323. BMJ Open Respir Res. 2025. PMID: 40216412 Free PMC article.
References
-
- Andrews, M.G., Mukhtar, T., Eze, U.C., Simoneau, C.R., Perez, Y., Mostajo-Radji, M.A., Wang, S., Velmeshev, D., Salma, J., Kumar, G.R., Pollen, A.A., Crouch, E.E., Ott, M., Kriegstein, A.R., 2021. Tropism of SARS-CoV-2 for developing human cortical astrocytes. bioRxiv: the preprint server for biology.
-
- Arthos J., Cicala C., Martinelli E., Macleod K., Van Ryk D., Wei D., Xiao Z., Veenstra T.D., Conrad T.P., Lempicki R.A., McLaughlin S., Pascuccio M., Gopaul R., McNally J., Cruz C.C., Censoplano N., Chung E., Reitano K.N., Kottilil S., Goode D.J., Fauci A.S. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008;9(3):301–309. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

