Immune Checkpoint Inhibition-Related Myasthenia-Myositis-Myocarditis Responsive to Complement Blockade
- PMID: 37884388
- PMCID: PMC10602369
- DOI: 10.1212/NXI.0000000000200177
Immune Checkpoint Inhibition-Related Myasthenia-Myositis-Myocarditis Responsive to Complement Blockade
Abstract
Objective: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy but come with immune-related adverse events (irAEs) that provide a novel challenge for treating physicians. Neuromuscular irAEs, including myositis, myasthenia gravis (MG), and demyelinating polyradiculoneuropathy, lead to significant morbidity and mortality.
Methods: We present a case of severe myasthenia-myositis-myocarditis overlap in a patient receiving ICIs for breast cancer. Clinical findings were recorded.
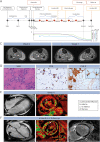
Results: A 47-year-old woman developed tetraparesis, dysphagia, and muscle pain during ICI treatment. MG with a thymoma had been diagnosed earlier. Neuromuscular overlap irAEs with cardiac affection was confirmed, and ICI treatment was discontinued. Given a lack of clinical response to standard therapies, a muscle biopsy was performed demonstrating complement deposition. Eculizumab treatment led to rapid improvement in muscle strength and cardiac function.
Discussion: Neuromuscular irAEs are associated with a high in-hospital mortality, and specific treatment strategies remain an unmet need. Here, early muscle biopsy enabled targeted therapy after standard approaches failed, thereby highlighting the value of identifying a specific treatment target. To improve therapeutic outcomes, the development of patient-tailored strategies for neuromuscular irAEs requires further studies.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
