Pseudomonas aeruginosa biofilm exopolysaccharides: assembly, function, and degradation
- PMID: 37884397
- PMCID: PMC10644985
- DOI: 10.1093/femsre/fuad060
Pseudomonas aeruginosa biofilm exopolysaccharides: assembly, function, and degradation
Abstract
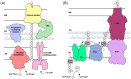
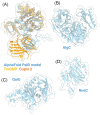
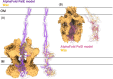
The biofilm matrix is a fortress; sheltering bacteria in a protective and nourishing barrier that allows for growth and adaptation to various surroundings. A variety of different components are found within the matrix including water, lipids, proteins, extracellular DNA, RNA, membrane vesicles, phages, and exopolysaccharides. As part of its biofilm matrix, Pseudomonas aeruginosa is genetically capable of producing three chemically distinct exopolysaccharides - alginate, Pel, and Psl - each of which has a distinct role in biofilm formation and immune evasion during infection. The polymers are produced by highly conserved mechanisms of secretion, involving many proteins that span both the inner and outer bacterial membranes. Experimentally determined structures, predictive modelling of proteins whose structures are yet to be solved, and structural homology comparisons give us insight into the molecular mechanisms of these secretion systems, from polymer synthesis to modification and export. Here, we review recent advances that enhance our understanding of P. aeruginosa multiprotein exopolysaccharide biosynthetic complexes, and how the glycoside hydrolases/lyases within these systems have been commandeered for antimicrobial applications.
Keywords: Pseudomonas aeruginosa; Pel; Psl; alginate; biofilm; biologics; exopolysaccharide; exopolysaccharide secretion system; glycoside hydrolases.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The authors declare no competing interests.
Figures









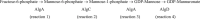
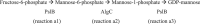
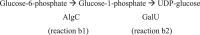
References
-
- Alipour M, Suntres ZE, Omri A. Importance of DNase and alginate lyase for enhancing free and liposome encapsulated aminoglycoside activity against Pseudomonas aeruginosa. J Antimicrob Chemother. 2009;64:317–25. - PubMed
-
- Alkawash MA, Soothill JS, Schiller NL. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Apmis. 2006;114:131–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

