Genetic interactions between polycystin-1 and Wwtr1 in osteoblasts define a novel mechanosensing mechanism regulating bone formation in mice
- PMID: 37884491
- PMCID: PMC10603112
- DOI: 10.1038/s41413-023-00295-4
Genetic interactions between polycystin-1 and Wwtr1 in osteoblasts define a novel mechanosensing mechanism regulating bone formation in mice
Abstract
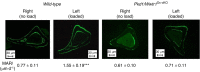
Molecular mechanisms transducing physical forces in the bone microenvironment to regulate bone mass are poorly understood. Here, we used mouse genetics, mechanical loading, and pharmacological approaches to test the possibility that polycystin-1 and Wwtr1 have interdependent mechanosensing functions in osteoblasts. We created and compared the skeletal phenotypes of control Pkd1flox/+;Wwtr1flox/+, Pkd1Oc-cKO, Wwtr1Oc-cKO, and Pkd1/Wwtr1Oc-cKO mice to investigate genetic interactions. Consistent with an interaction between polycystins and Wwtr1 in bone in vivo, Pkd1/Wwtr1Oc-cKO mice exhibited greater reductions of BMD and periosteal MAR than either Wwtr1Oc-cKO or Pkd1Oc-cKO mice. Micro-CT 3D image analysis indicated that the reduction in bone mass was due to greater loss in both trabecular bone volume and cortical bone thickness in Pkd1/Wwtr1Oc-cKO mice compared to either Pkd1Oc-cKO or Wwtr1Oc-cKO mice. Pkd1/Wwtr1Oc-cKO mice also displayed additive reductions in mechanosensing and osteogenic gene expression profiles in bone compared to Pkd1Oc-cKO or Wwtr1Oc-cKO mice. Moreover, we found that Pkd1/Wwtr1Oc-cKO mice exhibited impaired responses to tibia mechanical loading in vivo and attenuation of load-induced mechanosensing gene expression compared to control mice. Finally, control mice treated with a small molecule mechanomimetic, MS2 that activates the polycystin complex resulted in marked increases in femoral BMD and periosteal MAR compared to vehicle control. In contrast, Pkd1/Wwtr1Oc-cKO mice were resistant to the anabolic effects of MS2. These findings suggest that PC1 and Wwtr1 form an anabolic mechanotransduction signaling complex that mediates mechanical loading responses and serves as a potential novel therapeutic target for treating osteoporosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Genetic interactions between Polycystin-1 and TAZ in osteoblasts define a novel mechanosensing mechanism regulating bone formation in mice.Res Sq [Preprint]. 2023 May 29:rs.3.rs-2957026. doi: 10.21203/rs.3.rs-2957026/v1. Res Sq. 2023. Update in: Bone Res. 2023 Oct 26;11(1):57. doi: 10.1038/s41413-023-00295-4. PMID: 37398127 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

