RNA-based translation activators for targeted gene upregulation
- PMID: 37884512
- PMCID: PMC10603104
- DOI: 10.1038/s41467-023-42252-z
RNA-based translation activators for targeted gene upregulation
Abstract
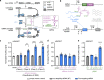
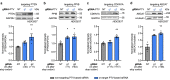
Technologies capable of programmable translation activation offer strategies to develop therapeutics for diseases caused by insufficient gene expression. Here, we present "translation-activating RNAs" (taRNAs), a bifunctional RNA-based molecular technology that binds to a specific mRNA of interest and directly upregulates its translation. taRNAs are constructed from a variety of viral or mammalian RNA internal ribosome entry sites (IRESs) and upregulate translation for a suite of target mRNAs. We minimize the taRNA scaffold to 94 nucleotides, identify two translation initiation factor proteins responsible for taRNA activity, and validate the technology by amplifying SYNGAP1 expression, a haploinsufficiency disease target, in patient-derived cells. Finally, taRNAs are suitable for delivery as RNA molecules by lipid nanoparticles (LNPs) to cell lines, primary neurons, and mouse liver in vivo. taRNAs provide a general and compact nucleic acid-based technology to upregulate protein production from endogenous mRNAs, and may open up possibilities for therapeutic RNA research.
© 2023. The Author(s).
Conflict of interest statement
Y.C. and B.C.D. have filed a patent for the taRNA technology (Patent Applicant: University of Chicago; Authors: Y.C. and B.C.D.; Serial number 17/905,116; Status: Pending). B.C.D. is a founder and holds equity in Tornado Bio, Inc. The remaining authors declare no competing interests related to this work.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

