Trim21 depletion alleviates bone loss in osteoporosis via activation of YAP1/β-catenin signaling
- PMID: 37884520
- PMCID: PMC10603047
- DOI: 10.1038/s41413-023-00296-3
Trim21 depletion alleviates bone loss in osteoporosis via activation of YAP1/β-catenin signaling
Abstract
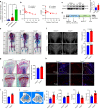
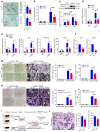
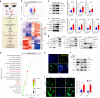
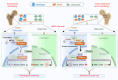
Despite the diverse roles of tripartite motif (Trim)-containing proteins in the regulation of autophagy, the innate immune response, and cell differentiation, their roles in skeletal diseases are largely unknown. We recently demonstrated that Trim21 plays a crucial role in regulating osteoblast (OB) differentiation in osteosarcoma. However, how Trim21 contributes to skeletal degenerative disorders, including osteoporosis, remains unknown. First, human and mouse bone specimens were evaluated, and the results showed that Trim21 expression was significantly elevated in bone tissues obtained from osteoporosis patients. Next, we found that global knockout of the Trim21 gene (KO, Trim21-/-) resulted in higher bone mass compared to that of the control littermates. We further demonstrated that loss of Trim21 promoted bone formation by enhancing the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and elevating the activity of OBs; moreover, Trim21 depletion suppressed osteoclast (OC) formation of RAW264.7 cells. In addition, the differentiation of OCs from bone marrow-derived macrophages (BMMs) isolated from Trim21-/- and Ctsk-cre; Trim21f/f mice was largely compromised compared to that of the littermate control mice. Mechanistically, YAP1/β-catenin signaling was identified and demonstrated to be required for the Trim21-mediated osteogenic differentiation of BMSCs. More importantly, the loss of Trim21 prevented ovariectomy (OVX)- and lipopolysaccharide (LPS)-induced bone loss in vivo by orchestrating the coupling of OBs and OCs through YAP1 signaling. Our current study demonstrated that Trim21 is crucial for regulating OB-mediated bone formation and OC-mediated bone resorption, thereby providing a basis for exploring Trim21 as a novel dual-targeting approach for treating osteoporosis and pathological bone loss.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

