Lineage specific extracellular vesicle-associated protein biomarkers for the early detection of high grade serous ovarian cancer
- PMID: 37884576
- PMCID: PMC10603107
- DOI: 10.1038/s41598-023-44050-5
Lineage specific extracellular vesicle-associated protein biomarkers for the early detection of high grade serous ovarian cancer
Abstract
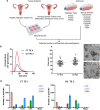
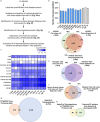
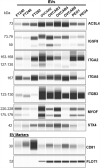
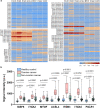
High grade serous ovarian carcinoma (HGSOC) accounts for ~ 70% of ovarian cancer cases. Non-invasive, highly specific blood-based tests for pre-symptomatic screening in women are crucial to reducing the mortality associated with this disease. Since most HGSOCs typically arise from the fallopian tubes (FT), our biomarker search focused on proteins found on the surface of extracellular vesicles (EVs) released by both FT and HGSOC tissue explants and representative cell lines. Using mass spectrometry, 985 EV proteins (exo-proteins) were identified that comprised the FT/HGSOC EV core proteome. Transmembrane exo-proteins were prioritized because these could serve as antigens for capture and/or detection. With a nano-engineered microfluidic platform, six newly discovered exo-proteins (ACSL4, IGSF8, ITGA2, ITGA5, ITGB3, MYOF) plus a known HGSOC associated protein, FOLR1 exhibited classification performance ranging from 85 to 98% in a case-control study using plasma samples representative of early (including stage IA/B) and late stage (stage III) HGSOCs. Furthermore, by a linear combination of IGSF8 and ITGA5 based on logistic regression analysis, we achieved a sensitivity of 80% with 99.8% specificity and a positive predictive value of 13.8%. Importantly, these exo-proteins also can accurately discriminate between ovarian and 12 types of cancers commonly diagnosed in women. Our studies demonstrate that these lineage-associated exo-biomarkers can detect ovarian cancer with high specificity and sensitivity early and potentially while localized to the FT when patient outcomes are more favorable.
© 2023. The Author(s).
Conflict of interest statement
A.K.G. is a co-founder of Sinochips Diagnostics, serves as a scientific advisory board member to Biovica, Clara Biotech, and Sinochips Diagnostics, and receives research funding from Predicine and VITRAC Therapeutics. Y.Z. serves as a scientific advisory board member to Clara Biotech. C.V.T., H.B.P, M.E.S. and A.K.G applied to patent ACSL4, IGSF8, ITGA2, ITGA5, ITGB3 and MYOF as early detection biomarkers for ovarian cancer. The other authors report no conflict of interest.
Figures


Update of
-
Lineage specific extracellular vesicle-associated protein biomarkers for the early detection of high grade serous ovarian cancer.Res Sq [Preprint]. 2023 May 3:rs.3.rs-2814022. doi: 10.21203/rs.3.rs-2814022/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Oct 26;13(1):18341. doi: 10.1038/s41598-023-44050-5. PMID: 37205573 Free PMC article. Updated. Preprint.
References
-
- van Nagell, J. R., Jr., Gallion, H. H., Pavlik, E. J. & DePriest, P. D. Ovarian cancer screening. Cancer76, 2086–2091 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

