Mitochondrial matrix RTN4IP1/OPA10 is an oxidoreductase for coenzyme Q synthesis
- PMID: 37884807
- PMCID: PMC10830421
- DOI: 10.1038/s41589-023-01452-w
Mitochondrial matrix RTN4IP1/OPA10 is an oxidoreductase for coenzyme Q synthesis
Abstract
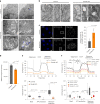
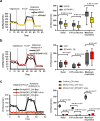
Targeting proximity-labeling enzymes to specific cellular locations is a viable strategy for profiling subcellular proteomes. Here, we generated transgenic mice (MAX-Tg) expressing a mitochondrial matrix-targeted ascorbate peroxidase. Comparative analysis of matrix proteomes from the muscle tissues showed differential enrichment of mitochondrial proteins. We found that reticulon 4-interacting protein 1 (RTN4IP1), also known as optic atrophy-10, is enriched in the mitochondrial matrix of muscle tissues and is an NADPH oxidoreductase. Interactome analysis and in vitro enzymatic assays revealed an essential role for RTN4IP1 in coenzyme Q (CoQ) biosynthesis by regulating the O-methylation activity of COQ3. Rtn4ip1-knockout myoblasts had markedly decreased CoQ9 levels and impaired cellular respiration. Furthermore, muscle-specific knockdown of dRtn4ip1 in flies resulted in impaired muscle function, which was reversed by dietary supplementation with soluble CoQ. Collectively, these results demonstrate that RTN4IP1 is a mitochondrial NAD(P)H oxidoreductase essential for supporting mitochondrial respiration activity in the muscle tissue.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












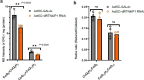
References
Publication types
MeSH terms
Substances
Grants and funding
- NRF-2022R1A2B5B03001658/National Research Foundation of Korea (NRF)
- NRF-2022R1C1C2004982/National Research Foundation of Korea (NRF)
- NRF-2017K1A1A2013124/National Research Foundation of Korea (NRF)
- HU20C0326/Korea Health Industry Development Institute (KHIDI)
- IBS-R008-D1/Institute for Basic Science (IBS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

