The impact of free antiretroviral therapy for pregnant non-citizens and their infants in Botswana
- PMID: 37885157
- PMCID: PMC10603275
- DOI: 10.1002/jia2.26161
The impact of free antiretroviral therapy for pregnant non-citizens and their infants in Botswana
Abstract
Introduction: In December 2019, the Botswana government expanded free antiretroviral therapy (ART) to include non-citizens. We evaluated the impact of this policy change on antenatal care (ANC), antiretroviral therapy coverage and adverse birth outcomes.
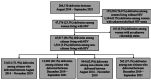
Methods: The Tsepamo Surveillance study collects data at up to 18 delivery sites in Botswana. We compared outcomes in citizens and non-citizens living with HIV before and after antiretroviral therapy expansion to non-citizens. Adverse birth outcomes included preterm delivery (PTD) <37 weeks, very preterm delivery (VPTD) <32 weeks, small for gestational age (SGA) <10th percentile, very small for gestational age (VSGA) <3rd percentile, stillbirth and neonatal death. Log-binomial regression models were constructed to generate risk ratios.
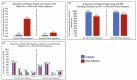
Results: From August 2014 to September 2021, 45,576 (96.5%) citizens and 1513 (3.2%) non-citizens living with HIV delivered; 954 (62.9%) non-citizen deliveries were before the antiretroviral therapy expansion, and 562 (37.1%) were after. Non-citizen ANC attendance among pregnant people living with HIV increased from 79.2% pre-expansion to 87.2% post-expansion (p<0.001), and became more similar to citizens (96.0% post-expansion). Non-citizens receiving any antenatal antiretroviral therapy increased from 65.5% pre-expansion to 89.9% post-expansion (p < 0.001), also more similar to citizens (97.2% post-expansion). Infants born to non-citizens with singleton gestations in the pre-expansion period had significantly greater risk of PTD (aRR = 1.28, 95% CI, 1.11, 1.46), VPTD (aRR = 1.89, 95% CI, 1.43, 2.44) and neonatal death (aRR = 1.69, 95% CI, 1.03, 2.60), but reduced SGA risk (aRR = 0.75; 95% CI, 0.62, 0.89) compared with citizens. Post-expansion, greater declines in most adverse outcomes were observed in non-citizens, with largely similar outcomes between non-citizens and citizens. Non-significant differences were observed for non-citizenship in PTD (aRR = 0.84, 95% CI, 0.66, 1.06), VPTD (aRR = 0.57, 95% CI, 0.28, 1.01), SGA (aRR = 0.91, 95% CI, 0.72, 1.13), VSGA (aRR = 0.87, 95% CI, 0.58, 1.25), stillbirth (aRR = 0.71, 95% CI, 0.35, 1.27) and neonatal death (aRR = 1.35, 95% CI, 0.60, 2.62).
Conclusions: Following the expansion of free antiretroviral therapy to non-citizens, gaps narrowed in ANC and antiretroviral therapy use in pregnancy between citizens and non-citizens living with HIV. Disparities in adverse birth outcomes were no longer observed.
Keywords: HIV; antiretroviral therapy; birth outcomes; botswana; non-citizen; pregnancy.
© 2023 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
There are no reported competing interests.
Figures

References
-
- Fiore S, Newell ML, Trabattoni D, Thorne C, Gray L, Savasi V, et al. Antiretroviral therapy‐associated modulation of Th1 and Th2 immune responses in HIV‐infected pregnant women. J Reprod Immunol. 2006;. 70(1–2):143–150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

