Bibliometric analysis of scientific papers on adverse reactions to COVID-19 vaccines published between 2019 and 2023
- PMID: 37885372
- PMCID: PMC10760317
- DOI: 10.1080/21645515.2023.2270194
Bibliometric analysis of scientific papers on adverse reactions to COVID-19 vaccines published between 2019 and 2023
Abstract
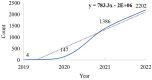
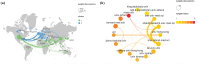
The Coronavirus Disease 2019 (COVID-19) pandemic has now persisted globally for four years, resulting in a staggering death toll of over 4 million individuals. The COVID-19 vaccine has emerged as a highly effective tool in controlling the spread of this virus. However, as the number of individuals receiving COVID-19. In this context, the investigation of adverse reactions related to COVID-19 vaccines holds paramount importance in relevant research. The purpose is to evaluate the current research status regarding adverse reactions associated with COVID-19 vaccines, offering insights for future research. A total of 3,746 articles were included in this analysis, and there has been a notable upward trajectory in the volume of published articles. The CiteSpace v6.1.R6, VOSviewer, SCImago Graphica, and Excel 2019 were employed to analyze and visualize the results. The institutions, countries, journals, authors, co-cited references, and keywords of these articles were analyzed. Furthermore, this study delves into the characteristics of articles on adverse reactions associated with COVID-19 vaccines. It was observed that the number of studies on COVID-19 vaccines has increased year by year since 2019 and witnessed a surge in output in 2021. The vast majority of studies have affirmed the overall safety of COVID-19 vaccines, with adverse reactions tending to be more concentrated in specific diseases. These findings provide valuable ideas for future research in this field and suggest the importance of strengthening international cooperation on adverse reactions to COVID-19 vaccines.
Keywords: COVID-19; Citespace; WoSCC; bibliometrics; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
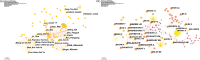




Similar articles
-
Global vaccine research and application hotspots and trends: a systematic bibliometric analysis based on SCIE highly cited papers.BMJ Open. 2025 Jan 22;15(1):e094935. doi: 10.1136/bmjopen-2024-094935. BMJ Open. 2025. PMID: 39843371 Free PMC article.
-
COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer.Biosci Trends. 2021 May 11;15(2):64-73. doi: 10.5582/bst.2021.01061. Epub 2021 Mar 19. Biosci Trends. 2021. PMID: 33746182 Review.
-
Knowledge mapping of global trends for myasthenia gravis development: A bibliometrics analysis.Front Immunol. 2023 Mar 2;14:1132201. doi: 10.3389/fimmu.2023.1132201. eCollection 2023. Front Immunol. 2023. PMID: 36936960 Free PMC article.
-
A bibliometric insight into nanomaterials in vaccine: trends, collaborations, and future avenues.Front Immunol. 2024 Aug 12;15:1420216. doi: 10.3389/fimmu.2024.1420216. eCollection 2024. Front Immunol. 2024. PMID: 39188723 Free PMC article.
-
Frontiers of monkeypox research: An analysis from the top 100 most influential articles in the field.Heliyon. 2023 Oct 2;9(10):e20566. doi: 10.1016/j.heliyon.2023.e20566. eCollection 2023 Oct. Heliyon. 2023. PMID: 37822624 Free PMC article. Review.
Cited by
-
Impact, Trends, and Visibility of Scientific Publications on Neurological Effects of COVID-19 Vaccine: A Scientometric Analysis.J Glob Infect Dis. 2025 Jun 26;17(2):98-103. doi: 10.4103/jgid.jgid_159_24. eCollection 2025 Apr-Jun. J Glob Infect Dis. 2025. PMID: 40727493 Free PMC article.
-
Mapping knowledge landscapes and emerging trends of Marburg virus: A text-mining study.Heliyon. 2024 Apr 15;10(8):e29691. doi: 10.1016/j.heliyon.2024.e29691. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38655363 Free PMC article.
References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19) a review. JAMA J Am Med Assoc. 2020;324:782–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
