Diastereodivergent Catalysis
- PMID: 37885579
- PMCID: PMC10598570
- DOI: 10.1021/jacsau.3c00216
Diastereodivergent Catalysis
Abstract
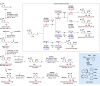
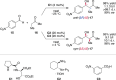
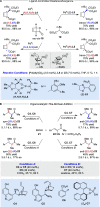
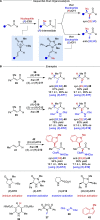
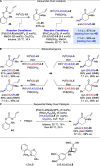
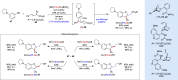
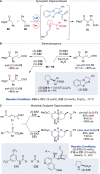
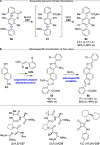
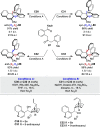
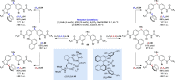
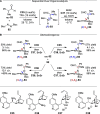
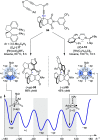
Alongside enantioselective catalysis, synthetic chemists are often confronted by the challenge of achieving catalyst control over the relative configuration to stereodivergently access desired diastereomers. Typically, these approaches iteratively or simultaneously control multiple stereogenic units for which dual catalytic methods comprising sequential, relay, and synergistic catalysis emerged as particularly efficient strategies. In this Perspective, the benefits and challenges of catalyst-controlled diastereodivergence in the construction of carbon stereocenters are discussed on the basis of illustrative examples. The concepts are then transferred to diastereodivergent catalysis for atropisomeric systems with twofold and higher-order stereogenicity as well as diastereodivergent catalyst control over E- and Z-configured alkenes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








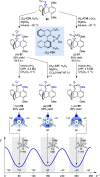
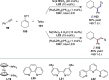
References
-
- Le Bel J. A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874, 22, 337–347.
-
- van ’t Hoff J. H. A suggestion looking to the extension into space of the structural formulas at present used in chemistry, and a note upon the relation between the optical activity and the chemical constitution of organic compounds. Arch. Neerl. Sci. Exactes Nat. 1874, 9, 445–454.
-
- Malakar C. C.; Dell’Amico L.; Zhang W. Dual Catalysis in Organic Synthesis: Current Challenges and New Trends. Eur. J. Org. Chem. 2023, 26, e20220111410.1002/ejoc.202201114. - DOI
-
- Martínez S.; Veth L.; Lainer B.; Dydio P. Challenges and Opportunities in Multicatalysis. ACS Catal. 2021, 11, 3891–3915. 10.1021/acscatal.0c05725. - DOI
Publication types
LinkOut - more resources
Full Text Sources
