Leishmania major- derived lipophosphoglycan influences the host's early immune response by inducing platelet activation and DKK1 production via TLR1/2
- PMID: 37885890
- PMCID: PMC10598878
- DOI: 10.3389/fimmu.2023.1257046
Leishmania major- derived lipophosphoglycan influences the host's early immune response by inducing platelet activation and DKK1 production via TLR1/2
Abstract
Background: Platelets are rapidly deployed to infection sites and respond to pathogenic molecules via pattern recognition receptors (TLR, NLRP). Dickkopf1 (DKK1) is a quintessential Wnt antagonist produced by a variety of cell types including platelets, endothelial cells, and is known to modulate pro-inflammatory responses in infectious diseases and cancer. Moreover, DKK1 is critical for forming leukocyte-platelet aggregates and induction of type 2 cell-mediated immune responses. Our previous publication showed activated platelets release DKK1 following Leishmania major recognition.
Results: Here we probed the role of the key surface virulence glycoconjugate lipophosphoglycan (LPG), on DKK1 production using null mutants deficient in LPG synthesis (Δlpg1- and Δlpg2-). Leishmania-induced DKK1 production was reduced to control levels in the absence of LPG in both mutants and was restored upon re-expression of the cognate LPG1 or LPG2 genes. Furthermore, the formation of leukocyte-platelet aggregates was dependent on LPG. LPG mediated platelet activation and DKK1 production occurs through TLR1/2.
Conclusion: Thus, LPG is a key virulence factor that induces DKK1 production from activated platelets, and the circulating DKK1 promotes Th2 cell polarization. This suggests that LPG-activated platelets can drive innate and adaptive immune responses to Leishmania infection.
Keywords: Leishmaniasis; P-selectin; innate response; leukocyte-platelet aggregates; platelet.
Copyright © 2023 Ihedioha, Sivakoses, Beverley, McMahon-Pratt and Bothwell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

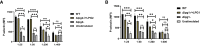

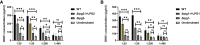



Similar articles
-
Leishmania major surface components and DKK1 signalling via LRP6 promote migration and longevity of neutrophils in the infection site.Front Immunol. 2024 Oct 22;15:1473133. doi: 10.3389/fimmu.2024.1473133. eCollection 2024. Front Immunol. 2024. PMID: 39502693 Free PMC article.
-
Toll-like receptor 2 (TLR2) plays a role in controlling cutaneous leishmaniasis in vivo, but does not require activation by parasite lipophosphoglycan.Parasit Vectors. 2016 Oct 6;9(1):532. doi: 10.1186/s13071-016-1807-8. Parasit Vectors. 2016. PMID: 27716391 Free PMC article.
-
Leishmania infantum Lipophosphoglycan-Deficient Mutants: A Tool to Study Host Cell-Parasite Interplay.Front Microbiol. 2018 Apr 5;9:626. doi: 10.3389/fmicb.2018.00626. eCollection 2018. Front Microbiol. 2018. PMID: 29675001 Free PMC article.
-
Leishmania lipophosphoglycan: how to establish structure-activity relationships for this highly complex and multifunctional glycoconjugate?Front Cell Infect Microbiol. 2015 Jan 21;4:193. doi: 10.3389/fcimb.2014.00193. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25653924 Free PMC article. Review.
-
Innate immune receptors in platelets and platelet-leukocyte interactions.J Leukoc Biol. 2020 Oct;108(4):1157-1182. doi: 10.1002/JLB.4MR0620-701R. Epub 2020 Aug 10. J Leukoc Biol. 2020. PMID: 32779243 Review.
Cited by
-
Leishmania major surface components and DKK1 signalling via LRP6 promote migration and longevity of neutrophils in the infection site.Front Immunol. 2024 Oct 22;15:1473133. doi: 10.3389/fimmu.2024.1473133. eCollection 2024. Front Immunol. 2024. PMID: 39502693 Free PMC article.
References
-
- Sheet WF. WHO Fact Sheet on Leishmaniasis . Geneva, Switzerland: World Health Organization; (Accessed July. 2021;10:2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

