Mild and scalable synthesis of phosphonorhodamines
- PMID: 37886078
- PMCID: PMC10599461
- DOI: 10.1039/d3sc02590j
Mild and scalable synthesis of phosphonorhodamines
Abstract
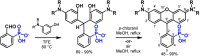
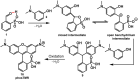
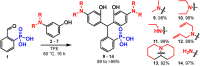
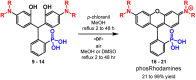
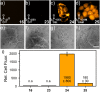
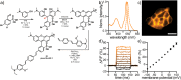
Since their discovery in 1887, rhodamines have become indispensable fluorophores for biological imaging. Recent studies have extensively explored heteroatom substitution at the 10' position and a variety of substitution patterns on the 3',6' nitrogens. Although 3-carboxy- and 3-sulfono-rhodamines were first reported in the 19th century, the 3-phosphono analogues have never been reported. Here, we report a mild, scalable synthetic route to 3-phosphonorhodamines. We explore the substrate scope and investigate mechanistic details of an exogenous acid-free condensation. Tetramethyl-3-phosphonorhodamine (phosTMR) derivatives can be accessed on the 1.5 mmol scale in up to 98% yield (2 steps). phosTMR shows a 12- to 500-fold increase in water solubility relative to 3-carboxy and 3-sulfonorhodamine derivatives and has excellent chemical stability. Additionally, phosphonates allow for chemical derivatization; esterification of phosTMR facilitates intracellular delivery with localization profiles that differ from 3-carboxyrhodamines. The free phosphonate can be incorporated into a molecular wire scaffold to create a phosphonated rhodamine voltage reporter, phosphonoRhoVR. PhosRhoVR 1 can be synthesized in just 6 steps, with an overall yield of 37% to provide >400 mg of material, compared to a 6-step, ∼2% yield for the previously reported RhoVR 1. PhosRhoVR 1 possesses excellent voltage sensitivity (37% ΔF/F) and a 2-fold increase in cellular brightness compared to RhoVR 1.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
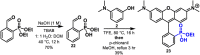

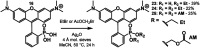
References
-
- Karstens T. Kobs K. Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 2002;84:1871–1872. doi: 10.1021/j100451a030. - DOI
-
- Grimm J. B. English B. P. Chen J. Slaughter J. P. Zhang Z. Revyakin A. Patel R. Macklin J. J. Normanno D. Singer R. H. Lionnet T. Lavis L. D. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods. 2015;12:244–250. doi: 10.1038/nmeth.3256. - DOI - PMC - PubMed

