Hypoxia releases S-nitrosocysteine from carotid body glomus cells-relevance to expression of the hypoxic ventilatory response
- PMID: 37886129
- PMCID: PMC10598756
- DOI: 10.3389/fphar.2023.1250154
Hypoxia releases S-nitrosocysteine from carotid body glomus cells-relevance to expression of the hypoxic ventilatory response
Abstract
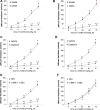
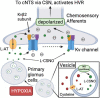
We have provided indirect pharmacological evidence that hypoxia may trigger release of the S-nitrosothiol, S-nitroso-L-cysteine (L-CSNO), from primary carotid body glomus cells (PGCs) of rats that then activates chemosensory afferents of the carotid sinus nerve to elicit the hypoxic ventilatory response (HVR). The objective of this study was to provide direct evidence, using our capacitive S-nitrosothiol sensor, that L-CSNO is stored and released from PGCs extracted from male Sprague Dawley rat carotid bodies, and thus further pharmacological evidence for the role of S-nitrosothiols in mediating the HVR. Key findings of this study were that 1) lysates of PGCs contained an S-nitrosothiol with physico-chemical properties similar to L-CSNO rather than S-nitroso-L-glutathione (L-GSNO), 2) exposure of PGCs to a hypoxic challenge caused a significant increase in S-nitrosothiol concentrations in the perfusate to levels approaching 100 fM via mechanisms that required extracellular Ca2+, 3) the dose-dependent increases in minute ventilation elicited by arterial injections of L-CSNO and L-GSNO were likely due to activation of small diameter unmyelinated C-fiber carotid body chemoafferents, 4) L-CSNO, but not L-GSNO, responses were markedly reduced in rats receiving continuous infusion (10 μmol/kg/min, IV) of both S-methyl-L-cysteine (L-SMC) and S-ethyl-L-cysteine (L-SEC), 5) ventilatory responses to hypoxic gas challenge (10% O2, 90% N2) were also due to the activation of small diameter unmyelinated C-fiber carotid body chemoafferents, and 6) the HVR was markedly diminished in rats receiving L-SMC plus L-SEC. This data provides evidence that rat PGCs synthesize an S-nitrosothiol with similar properties to L-CSNO that is released in an extracellular Ca2+-dependent manner by hypoxia.
Keywords: L-S-nitrosoglutathione; S-ethyl-L-cysteine; S-nitroso-L-cysteine; Smethyl-L-cysteine; minute ventilation; primary glomus cells.
Copyright © 2023 Seckler, Getsy, May, Gaston, Baby, Lewis, Bates and Lewis.
Conflict of interest statement
SB was employed by Galleon Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

