Irradiation Attenuates Systemic Lupus Erythematosus-Like Morbidity in NZBWF1 Mice: Focusing on CD180-Negative Cells
- PMID: 37886369
- PMCID: PMC10599955
- DOI: 10.1155/2023/9969079
Irradiation Attenuates Systemic Lupus Erythematosus-Like Morbidity in NZBWF1 Mice: Focusing on CD180-Negative Cells
Abstract
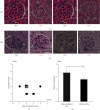
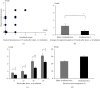
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the production of autoantibodies that can induce systemic inflammation. Ultraviolet-A and X-ray irradiation have been reported to have therapeutic effects in patients with SLE. We previously demonstrated that CD180-negative cells, these are radiosensitive, contribute to the development of SLE-like morbidity in NZBWF1 mice. In this study, the effects of irradiation on SLE-like morbidity manifestations in NZBWF1 mice and on CD180-negative cells were investigated. Whole-body irradiation, excluding the head, attenuated SLE-like morbidity in vivo, as indicated by the prevention of the renal lesion development, inhibition of anti-dsDNA antibody production, reduction of urinary protein levels, and prolongation of the lifespan. Irradiation also reduced the proportion of CD180-negative cells in the spleen. Although other immune cells or molecules may be triggered because of the whole-body irradiation treatment, previous research, and the current results suggest a strong relationship between the radiation-induced decrease in CD180-negative cells and the amelioration of SLE-like morbidities. Clinical trials assessing CD180-negative cells as a therapeutic target for SLE have been hampered by the lack of validated cell markers; nonetheless, the present findings suggest that radiotherapy may be a new therapeutic strategy for managing SLE symptoms.
Copyright © 2023 Kazuko Fujita et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



Similar articles
-
Pathogenesis of lupus-like nephritis through autoimmune antibody produced by CD180-negative B lymphocytes in NZBWF1 mouse.Immunol Lett. 2012 May 30;144(1-2):1-6. doi: 10.1016/j.imlet.2012.02.012. Epub 2012 Feb 28. Immunol Lett. 2012. PMID: 22387632
-
Ligation of CD180 inhibits IFN-α signaling in a Lyn-PI3K-BTK-dependent manner in B cells.Cell Mol Immunol. 2017 Feb;14(2):192-202. doi: 10.1038/cmi.2015.61. Epub 2015 Aug 17. Cell Mol Immunol. 2017. PMID: 26277892 Free PMC article.
-
CD180 Ligation Inhibits TLR7- and TLR9-Mediated Activation of Macrophages and Dendritic Cells Through the Lyn-SHP-1/2 Axis in Murine Lupus.Front Immunol. 2018 Nov 15;9:2643. doi: 10.3389/fimmu.2018.02643. eCollection 2018. Front Immunol. 2018. PMID: 30498494 Free PMC article.
-
RP105-negative B cells in systemic lupus erythematosus.Clin Dev Immunol. 2012;2012:259186. doi: 10.1155/2012/259186. Epub 2011 Sep 15. Clin Dev Immunol. 2012. PMID: 21941580 Free PMC article. Review.
-
[BCMA and autoantibody-producing RP105 B cells; possible new targets of B cell therapy in systemic lupus erythematosus].Nihon Rinsho Meneki Gakkai Kaishi. 2012;35(1):38-45. doi: 10.2177/jsci.35.38. Nihon Rinsho Meneki Gakkai Kaishi. 2012. PMID: 22374441 Review. Japanese.
References
-
- McGrath H., Jr Ultraviolet-A1 irradiation decreases clinical disease activity and autoantibodies in patients with systemic lupus erythematosus. Clinical and Experimental Rheumatology . 1994;12(2):129–135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

