This is a preprint.
Ancient origin of the rod bipolar cell pathway in the vertebrate retina
- PMID: 37886445
- PMCID: PMC10602083
- DOI: 10.21203/rs.3.rs-3411693/v1
Ancient origin of the rod bipolar cell pathway in the vertebrate retina
Update in
-
Ancient origin of the rod bipolar cell pathway in the vertebrate retina.Nat Ecol Evol. 2024 Jun;8(6):1165-1179. doi: 10.1038/s41559-024-02404-w. Epub 2024 Apr 16. Nat Ecol Evol. 2024. PMID: 38627529
Abstract
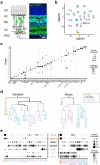
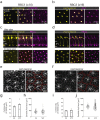
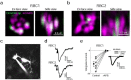
Vertebrates rely on rod photoreceptors for vision in low-light conditions. Mammals have a specialized downstream circuit for rod signaling called the primary rod pathway, which comprises specific cell types and wiring patterns that are thought to be unique to this lineage. Thus, it has been long assumed that the primary rod pathway evolved in mammals. Here, we challenge this view by demonstrating that the mammalian primary rod pathway is conserved in zebrafish, which diverged from extant mammals ~400 million years ago. Using single-cell RNA-sequencing, we identified two bipolar cell (BC) types in zebrafish that are related to mammalian rod BCs (RBCs) of the primary rod pathway. By combining electrophysiology, histology, and ultrastructural reconstruction of the zebrafish RBCs, we found that, like mammalian RBCs, both zebrafish RBC types connect with all rods in their dendritic territory, and provide output largely onto amacrine cells. The wiring pattern of the amacrine cells post-synaptic to one RBC type is strikingly similar to that of mammalian RBCs, suggesting that the cell types and circuit design of the primary rod pathway have emerged before the divergence of teleost fish and amniotes. The second RBC type, which forms separate pathways, is either lost in mammals or emerged in fish.
Keywords: Evolution; Retina; Rod; Rod bipolar cells.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures



References
-
- Field G. D. & Rieke F. Nonlinear Signal Transfer from Mouse Rods to Bipolar Cells and Implications for Visual Sensitivity. Neuron 34, 773–785 (2002). - PubMed
-
- Field G. D. & Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron 35, 733–747 (2002). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

