This is a preprint.
Dapagliflozin cardiovascular effects on end-stage kidney disease (DARE-ESKD-2) trial: rationale and design
- PMID: 37886458
- PMCID: PMC10602138
- DOI: 10.21203/rs.3.rs-3434207/v1
Dapagliflozin cardiovascular effects on end-stage kidney disease (DARE-ESKD-2) trial: rationale and design
Update in
-
Dapagliflozin cardiovascular effects on end-stage kidney disease (DARE-ESKD-2) trial: rationale and design.Expert Opin Drug Saf. 2025 Dec;24(12):1471-1477. doi: 10.1080/14740338.2024.2412228. Epub 2024 Oct 13. Expert Opin Drug Saf. 2025. PMID: 39377184
Abstract
Purpose: Sodium glucose co-transporter 2 inhibitors (SGLT2i) remarkably reduced the incidence of hospitalization for heart failure and cardiovascular death of conservatively managed chronic kidney disease. We hypothesized that adding SGLT2i to standard treatment would yield cardiovascular benefits also in end-stage kidney disease (ESKD) individuals on dialysis.
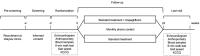
Methods: The DARE-ESKD-2 Trial (NCT05685394) is an ongoing, single-center, open-label, controlled trial aimed at assessing the cardiovascular effects of dapagliflozin in ESKD on dialysis. Eligible patients are adults on renal replacement therapy for more than 3 prior to enrollment. Exclusion criteria encompass pregnancy, liver failure, and current use of a SGLT2i. After signing an informed consent form, participants are randomized 1:1 to either dapagliflozin 10mg PO plus standard treatment or standard treatment alone for 6 months. Echocardiogram, anthropometry, blood sample collection, 6-min walk test, gait speed, and Kansas City Cardiomyopathy Questionnaire (KCCQ), are performed at baseline and at study termination. Participants are contacted monthly during treatment for outcomes disclosure. The primary endpoint of our study is the between-groups differences in posttreatment changes in plasma levels of N-terminal pro-B natriuretic peptide. Secondary endpoints include the differences between groups in the changes of echocardiography measurements, cardiopulmonary tests performance, body composition. The incidence of safety endpoints will also be diligently compared between study arms.
Conclusion: The DARE-ESKD-2 trial will provide unprecedented data on the cardiovascular safety and efficacy of SGLT2i in ESKD individuals on dialysis. This study will pave the grounds for improving clinical outcomes of dialysis recipients.
Conflict of interest statement
Declaration of Competing Interest Authors declare that they have no competing interest in this manuscript.
References
-
- Nuffield Department of Population Health Renal Studies G, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–801. 10.1016/S0140-6736(22)02074-8. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources