This is a preprint.
Cell-Free DNA Methylation Analysis as a Marker of Malignancy in Pleural Fluid
- PMID: 37886511
- PMCID: PMC10602127
- DOI: 10.21203/rs.3.rs-3390107/v1
Cell-Free DNA Methylation Analysis as a Marker of Malignancy in Pleural Fluid
Update in
-
Cell-free DNA methylation analysis as a marker of malignancy in pleural fluid.Sci Rep. 2024 Feb 5;14(1):2939. doi: 10.1038/s41598-024-53132-x. Sci Rep. 2024. PMID: 38316884 Free PMC article.
Abstract
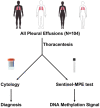
Background: Diagnosis of malignant pleural effusion (MPE) is made by cytological examination of pleural fluid or histological examination of pleural tissue from biopsy. Unfortunately, detection of malignancy using cytology has an overall sensitivity of 50%, and is dependent upon tumor load, volume of fluid assessed, and cytopathologist experience. The diagnostic yield of pleural fluid cytology is also compromised by low abundance of tumor cells or when morphology is obscured by inflammation or reactive mesothelial cells. A reliable molecular marker that may complement fluid cytology malignant pleural effusion diagnosis is needed. The purpose of this study was to establish a molecular diagnostic approach based on pleural effusion cell-free DNA methylation analysis for the differential diagnosis of malignant pleural effusion and benign pleural effusion.
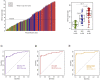
Results: This was a blind, prospective case-control biomarker study. We recruited 104 patients with pleural effusion for the study. We collected pleural fluid from patients with: MPE (n = 48), PPE (n = 28), and benign PE (n = 28), and performed the Sentinel-MPE liquid biopsy assay. The methylation level of Sentinel-MPE was markedly higher in the MPE samples compared to BPE control samples (p < 0.0001) and the same tendency was observed relative to PPE (p = 0.004). We also noted that the methylation signal was significantly higher in PPE relative to BPE (p < 0.001). We also assessed the diagnostic efficiency of the Sentinel-MPE test by performing receiver operating characteristic analysis (ROC). For the ROC analysis we combined the malignant and paramalignant groups (n = 76) and compared against the benign group (n = 28). The detection sensitivity and specificity of the Sentinel-MPE test was high (AUC = 0.912). The Sentinel-MPE appears to have better performance characteristics than cytology analysis. However, combining Sentinel-MPE with cytology analysis could be an even more effective approach for the diagnosis of MPE.
Conclusions: The Sentinel-MPE test can discriminate between BPE and MPE. The Sentinel-MPE liquid biopsy test can detect aberrant DNA in several different tumor types. The Sentinel-MPE test can be a complementary tool to cytology in the diagnosis of MPE.
Conflict of interest statement
Competing interests MN and BF have disclosed an outside interest in Precision Epigenomics, Inc to the University of Arizona. Conflicts of interest resulting from this interest are being managed by The University of Arizona in accordance with its policies.
Figures
References
-
- Semaan R, Feller-Kopman D, Slatore C, Sockrider M. Malignant Pleural Effusions. Am J Respir Crit Care Med 2016;194:P11–2. - PubMed
-
- Bashour SI, Mankidy BJ, Lazarus DR. Update on the diagnosis and management of malignant pleural effusions. Respir Med 2022;196:106802. - PubMed
-
- Jiang B, Li XL, Yin Y, et al. Ultrasound elastography: a novel tool for the differential diagnosis of pleural effusion. Eur Respir J 2019;54. - PubMed
-
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997;10:1907–13. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

