This is a preprint.
Neutrophil swarms containing myeloid-derived suppressor cells are crucial for limiting oral mucosal infection by C. albicans
- PMID: 37886517
- PMCID: PMC10602121
- DOI: 10.21203/rs.3.rs-3346012/v1
Neutrophil swarms containing myeloid-derived suppressor cells are crucial for limiting oral mucosal infection by C. albicans
Update in
-
Neutrophil swarming is crucial for limiting oral mucosal infection by Candida albicans.J Leukoc Biol. 2025 Mar 14;117(3):qiae239. doi: 10.1093/jleuko/qiae239. J Leukoc Biol. 2025. PMID: 39530591 Free PMC article.
Abstract
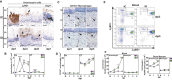
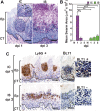
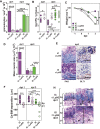
Oral mucosal colonization by C. albicans (Ca) is benign in healthy people but progresses to deeper infection known as oropharyngeal candidiasis (OPC) that may become disseminated when combined with immunosuppression. Cortisone-induced immunosuppression is a well-known risk factor for OPC, however the mechanism by which it permits infection is poorly understood. Neutrophils are the primary early sentinels preventing invasive fungal growth, and here we identify that in vivo neutrophil functional complexes known as swarms are crucial for preventing Ca invasion which are disrupted by cortisone. Neutrophil swarm function required leukotriene B4 receptor 1 (BLT1) expression, and swarms were further characterized by peripheral association of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) showing that OPC recruits PMN-MDSCs to this site of infection. Furthermore, PMN-MDSCs associated with Ca hyphae had no direct antifungal effect but showed prolonged survival times and increased autophagy. Thus in vivo neutrophil swarms are complex structures with spatially associated PMN-MDSCs that likely contribute immunoregulatory functions to resolve OPC. These swarm structures have an important function in preventing deep invasion by Ca within the oral mucosa and represent a mechanism for increased disease severity under immune deficient clinical settings.
Conflict of interest statement
Competing Interests. None
Figures




References
-
- Mosci P., et al.: Involvement of IL-17A in preventing the development of deep-seated candidiasis from oropharyngeal infection. Microbes Infect. 16, 678–689 (2014) - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

