Bone Involvement in Rheumatoid Arthritis and Spondyloartritis: An Updated Review
- PMID: 37887030
- PMCID: PMC10604370
- DOI: 10.3390/biology12101320
Bone Involvement in Rheumatoid Arthritis and Spondyloartritis: An Updated Review
Abstract
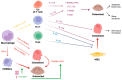
Several rheumatologic diseases are primarily distinguished by their involvement of bone tissue, which not only serves as a mere target of the condition but often plays a pivotal role in its pathogenesis. This scenario is particularly prominent in chronic inflammatory arthritis such as rheumatoid arthritis (RA) and spondyloarthritis (SpA). Given the immunological and systemic nature of these diseases, in this review, we report an overview of the pathogenic mechanisms underlying specific bone involvement, focusing on the complex interactions that occur between bone tissue's own cells and the molecular and cellular actors of the immune system, a recent and fascinating field of interest defined as osteoimmunology. Specifically, we comprehensively elaborate on the distinct pathogenic mechanisms of bone erosion seen in both rheumatoid arthritis and spondyloarthritis, as well as the characteristic process of aberrant bone formation observed in spondyloarthritis. Lastly, chronic inflammatory arthritis leads to systemic bone involvement, resulting in systemic bone loss and consequent osteoporosis, along with increased skeletal fragility.
Keywords: bone tissue; erosions; new bone formation; osteoimmunology; rheumatoid arthritis; spondyloarthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

