Analytical Validation of a Spiral Microfluidic Chip with Hydrofoil-Shaped Pillars for the Enrichment of Circulating Tumor Cells
- PMID: 37887131
- PMCID: PMC10605072
- DOI: 10.3390/bios13100938
Analytical Validation of a Spiral Microfluidic Chip with Hydrofoil-Shaped Pillars for the Enrichment of Circulating Tumor Cells
Abstract
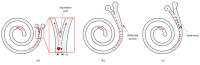
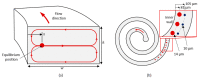
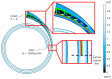
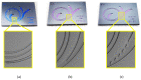
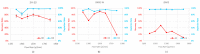
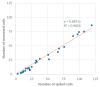
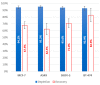
The isolation of circulating tumor cells (CTCs) from peripheral blood with high efficiency remains a challenge hindering the utilization of CTC enrichment methods in clinical practice. Here, we propose a microfluidic channel design for the size-based hydrodynamic enrichment of CTCs from blood in an epitope-independent and high-throughput manner. The microfluidic channel comprises a spiral-shaped part followed by a widening part, incorporating successive streamlined pillars, that improves the enrichment efficiency. The design was tested against two benchmark designs, a spiral microfluidic channel and a spiral microfluidic channel followed by a widening channel without the hydrofoils, by processing 5 mL of healthy blood samples spiked with 100 MCF-7 cells. The results proved that the design with hydrofoil-shaped pillars perform significantly better in terms of recovery (recovery rate of 67.9% compared to 23.6% in spiral and 56.7% in spiral with widening section), at a cost of slightly lower white blood cell (WBC) depletion (depletion rate of 94.2% compared to 98.6% in spiral and 94.2% in spiral with widening section), at 1500 µL/min flow rate. For analytical validation, the design was further tested with A549, SKOV-3, and BT-474 cell lines, yielding recovery rates of 62.3 ± 8.4%, 71.0 ± 6.5%, and 82.9 ± 9.9%, respectively. The results are consistent with the size and deformability variation in the respective cell lines, where the increasing size and decreasing deformability affect the recovery rate in a positive manner. The analysis before and after the microfluidic chip process showed that the process does not affect cell viability.
Keywords: circulating tumor cell (CTC) separation; computational fluid dynamics; inertial hydrodynamics; microfluidic channel.
Conflict of interest statement
Ender Yildirim and Ozge Zorlu have a patent titled “Method of microfluidic particle separation enhancement and the device thereof” issued to Mikro Biyosistemler Elektronik Sanayi ve Ticaret A.Ş. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

