Neuroprotection Is in the Air-Inhaled Gases on Their Way to the Neurons
- PMID: 37887324
- PMCID: PMC10605176
- DOI: 10.3390/cells12202480
Neuroprotection Is in the Air-Inhaled Gases on Their Way to the Neurons
Abstract
Cerebral injury is a leading cause of long-term disability and mortality. Common causes include major cardiovascular events, such as cardiac arrest, ischemic stroke, and subarachnoid hemorrhage, traumatic brain injury, and neurodegenerative as well as neuroinflammatory disorders. Despite improvements in pharmacological and interventional treatment options, due to the brain's limited regeneration potential, survival is often associated with the impairment of crucial functions that lead to occupational inability and enormous economic burden. For decades, researchers have therefore been investigating adjuvant therapeutic options to alleviate neuronal cell death. Although promising in preclinical studies, a huge variety of drugs thought to provide neuroprotective effects failed in clinical trials. However, utilizing medical gases, noble gases, and gaseous molecules as supportive treatment options may offer new perspectives for patients suffering neuronal damage. This review provides an overview of current research, potentials and mechanisms of these substances as a promising therapeutic alternative for the treatment of cerebral injury.
Keywords: argon; carbon monoxide; desflurane; helium; hydrogen sulfide; ischemia/reperfusion injury; isoflurane; neon; neuronal cell death; noble gases; sevoflurane; volatile anesthetics; xenon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
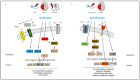
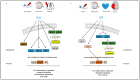
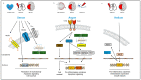
References
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

