SLC38A5 Modulates Ferroptosis to Overcome Gemcitabine Resistance in Pancreatic Cancer
- PMID: 37887353
- PMCID: PMC10605569
- DOI: 10.3390/cells12202509
SLC38A5 Modulates Ferroptosis to Overcome Gemcitabine Resistance in Pancreatic Cancer
Abstract
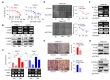
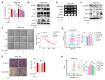
Pancreatic cancer is characterized by a poor prognosis, with its five-year survival rate lower than that of any other cancer type. Gemcitabine, a standard treatment for pancreatic cancer, often has poor outcomes for patients as a result of chemoresistance. Therefore, novel therapeutic targets must be identified to overcome gemcitabine resistance. Here, we found that SLC38A5, a glutamine transporter, is more highly overexpressed in gemcitabine-resistant patients than in gemcitabine-sensitive patients. Furthermore, the deletion of SLC38A5 decreased the proliferation and migration of gemcitabine-resistant PDAC cells. We also found that the inhibition of SLC38A5 triggered the ferroptosis signaling pathway via RNA sequencing. Also, silencing SLC38A5 induced mitochondrial dysfunction and reduced glutamine uptake and glutathione (GSH) levels, and downregulated the expressions of GSH-related genes NRF2 and GPX4. The blockade of glutamine uptake negatively modulated the mTOR-SREBP1-SCD1 signaling pathway. Therefore, suppression of SLC38A5 triggers ferroptosis via two pathways that regulate lipid ROS levels. Similarly, we observed that knockdown of SLC38A5 restored gemcitabine sensitivity by hindering tumor growth and metastasis in the orthotopic mouse model. Altogether, our results demonstrate that SLC38A5 could be a novel target to overcome gemcitabine resistance in PDAC therapy.
Keywords: PDAC; SLC38A5; ferroptosis; gemcitabine resistance; lipid ROS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

