Effectiveness of Immune Checkpoint Inhibitor with Anti-PD-1 Monotherapy or in Combination with Ipilimumab in Younger versus Older Adults with Advanced Melanoma
- PMID: 37887546
- PMCID: PMC10605250
- DOI: 10.3390/curroncol30100646
Effectiveness of Immune Checkpoint Inhibitor with Anti-PD-1 Monotherapy or in Combination with Ipilimumab in Younger versus Older Adults with Advanced Melanoma
Abstract
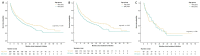
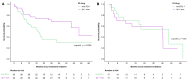
Background: The majority of melanoma is diagnosed in individuals between 55 and 84 years old. Current data varied in reporting differences in survival outcomes amongst different age groups. Methods: A retrospective, multi-center, provincial cohort database was used to investigate the relationship between age (<65 or ≥65 years old) and overall survival. Patients must have had histologically confirmed locally advanced or metastatic melanoma and had to have received at least one cycle of immunotherapy (single agent nivolumab, pembrolizumab, or combination ipilimumab plus nivolumab). Results: From August 2013 to May 2020, we identified 497 patients (median age = 64 [range 12-96 years]; 65.2% men; 36.4% with a BRAF mutation (V600E and V600K)). Of these, 260 were < 65 years old, and 237 were ≥65 years old. A total of 39.1% of the patients in the younger cohort received combination ICI compared with 10.2% in the older cohort, and the difference was statistically significant. Median survival amongst individuals aged ≥65 years old was shorter compared to individuals <65 years old, with a median overall survival of 17.1 (95% CI 12.3-22.9 months) months and 22.2 months (95% CI 18.7-33.8 months), respectively (p = 0.04), at a median follow-up of 34.4 months (range: 1.84-81.4 months). The survival difference was present in the cutaneous melanoma cohort where median OS was 18.2 months (95% CI 12.3-30.4 months) in patients ≥65 years old and 23.8 months (95% CI 19.2-48.2 months) in patients <65 years old, p = 0.04. There were no significant differences by age in the non-cutaneous melanoma cohort. A combination of nivolumab plus ipilimumab was associated with an improved overall survival hazard ratio of 0.48 (95% CI 0.36-0.65) as compared to anti-PD-1 monotherapy alone (p < 0.001). In the cutaneous cohort treated with anti-PD-1 monotherapy (n = 306), no significant differences were seen with median OS at 16.1 months (95% CI 11.4-25.7 months) in patients ≥65 years old and 17.1 months (95% CI 12.0-22.2 months) in patients <65 years old (p = 0.84). Tumor response to anti-PD-1 was higher in the older patients compared with the response in younger patients with cutaneous melanoma. Conclusions: Older melanoma patients have similar survival compared with younger patients after receiving the same treatment with anti-PD-1 monotherapy. The superior survival observed in the younger patients is possibly related to the higher utilization of combination ICI. Tumor response to immunotherapy is superior in older patients with cutaneous melanoma; however, younger patients may improve their survival by using combination ICI.
Keywords: age; immune checkpoint inhibitor; ipilimumab plus nivolumab; melanoma; nivolumab; older adults; pembrolizumab; survival.
Conflict of interest statement
T.C. received research funding from Pfizer. DYCH acted in a consulting or advisory role for Pfizer, Novartis, Bristol Myers Squib, Janssen, Astellas, Ipsen, Eisai, and Merck with research funding from Pfizer, Novartis, Exelixis, Bristol Myers Squibb, and Ipsen. All other authors have nothing to disclose.
Figures
References
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

