Conversion in a Resectable Tumor after Denosumab Neoadjuvant in a Large Dorsal Giant Cells Tumor: A Case Report and a Literature Review
- PMID: 37887575
- PMCID: PMC10605573
- DOI: 10.3390/curroncol30100675
Conversion in a Resectable Tumor after Denosumab Neoadjuvant in a Large Dorsal Giant Cells Tumor: A Case Report and a Literature Review
Abstract
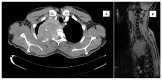
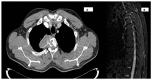
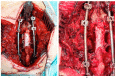
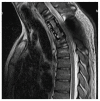
Giant cell tumors of bone are a rare entity, usually occurring in young patients and characteristically arising in the long bones. The spinal location is rare and usually presents with pain and/or neurological symptoms. The treatment of choice is surgery. Treatment with Denosumab, a bisphosphonate inhibitor of RANK-L, which is highly expressed in these tumors, has shown extensive activity in unresectable patients or those undergoing incomplete surgery. Preoperative treatment with this drug is gaining increasing interest, as its high potency in tumor reduction in this subtype of neoplasm has allowed resectability in selected patients. We present the case of a young patient with a large spinal tumor who, after neoadjuvant Denosumab, underwent complete en bloc surgery with clean margins and a great pathological response.
Keywords: bone giant cell tumor; denosumab; neoadjuvant.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

