Focal Adhesion Kinase Binds to the HPV E2 Protein to Regulate Initial Replication after Infection
- PMID: 37887719
- PMCID: PMC10609836
- DOI: 10.3390/pathogens12101203
Focal Adhesion Kinase Binds to the HPV E2 Protein to Regulate Initial Replication after Infection
Abstract
Human papillomaviruses are small DNA tumor viruses that infect cutaneous and mucosal epithelia. The viral lifecycle is linked to the differentiation status of the epithelium. During initial viral infection, the genomes replicate at a low copy number but the mechanism(s) the virus uses to control the copy number during this stage is not known. In this study, we demonstrate that the tyrosine kinase focal adhesion kinase (FAK) binds to and phosphorylates the high-risk viral E2 protein, the key regulator of HPV replication. The depletion of FAK with a specific PROTAC had no effect on viral DNA content in keratinocytes that already maintain HPV-16 and HPV-31 episomes. In contrast, the depletion of FAK significantly increased HPV-16 DNA content in keratinocytes infected with HPV-16 quasiviruses. These data imply that FAK prevents the over-replication of the HPV genome after infection through the interaction and phosphorylation of the E2 protein.
Keywords: HPV; focal adhesion kinase; replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

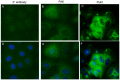
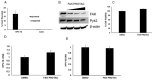

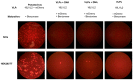
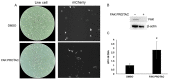
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

