Immunohistochemical Characterization of M1, M2, and M4 Macrophages in Leprosy Skin Lesions
- PMID: 37887741
- PMCID: PMC10610015
- DOI: 10.3390/pathogens12101225
Immunohistochemical Characterization of M1, M2, and M4 Macrophages in Leprosy Skin Lesions
Abstract
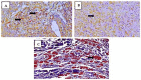
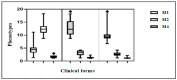
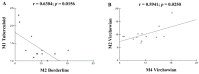
Mycobacterium leprae is the etiological agent of leprosy. Macrophages (Mφs) are key players involved in the pathogenesis of leprosy. In this study, immunohistochemical analysis was performed to examine the phenotype of Mφ subpopulations, namely M1, M2, and M4, in the skin lesions of patients diagnosed with leprosy. Based on the database of treatment-naïve patients treated between 2015 and 2019 at the Department of Dermatology of the University of the State of Pará, Belém, routine clinical screening samples were identified. The monolabeling protocol was used for M1 macrophages (iNOS, IL-6, TNF-α) and M2 macrophages (IL-10, IL-13, CD163, Arginase 1, TGF-β, FGFb), and the double-labeling protocol was used for M4 macrophages (IL-6, MMP7, MRP8, TNF-α e CD68). To confirm the M4 macrophage lineage, double labeling of the monoclonal antibodies CD68 and MRP8 was also performed. Our results demonstrated a statistically significant difference for the M1 phenotype among the Virchowian (VV) (4.5 ± 1.3, p < 0.0001), Borderline (1.6 ± 0.4, p < 0.0001), and tuberculoid (TT) (12.5 ± 1.8, p < 0.0001) clinical forms of leprosy. Additionally, the M2 phenotype showed a statistically significant difference among the VV (12.5 ± 2.3, p < 0.0001), Borderline (1.3 ± 0.2, p < 0.0001), and TT (3.2 ± 0.7, p < 0.0001) forms. For the M4 phenotype, a statistically significant difference was observed in the VV (9.8 ± 1.7, p < 0.0001), Borderline (1.2 ± 0.2, p < 0.0001), and TT (2.6 ± 0.7, p < 0.0001) forms. A significant correlation was observed between the VV M1 and M4 (r = 0.8712; p = 0.0000) and between the VV M2 × TT M1 (r = 0.834; p = 0.0002) phenotypes. The M1 Mφs constituted the predominant Mφ subpopulation in the TT and Borderline forms of leprosy, whereas the M2 Mφs showed increased immunoexpression and M4 was the predominant Mφ phenotype in VV leprosy. These results confirm the relationship of the Mφ profile with chronic pathological processes of the inflammatory response in leprosy.
Keywords: immunohistochemistry; immunology; leprosy; macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

