Statins and Cardiomyocyte Metabolism, Friend or Foe?
- PMID: 37887864
- PMCID: PMC10607220
- DOI: 10.3390/jcdd10100417
Statins and Cardiomyocyte Metabolism, Friend or Foe?
Abstract
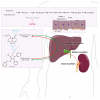
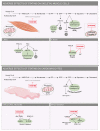
Statins inhibit HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis, and are the cornerstone of lipid-lowering treatment. They significantly reduce cardiovascular morbidity and mortality. However, musculoskeletal symptoms are observed in 7 to 29 percent of all users. The mechanism underlying these complaints has become increasingly clear, but less is known about the effect on cardiac muscle function. Here we discuss both adverse and beneficial effects of statins on the heart. Statins exert pleiotropic protective effects in the diseased heart that are independent of their cholesterol-lowering activity, including reduction in hypertrophy, fibrosis and infarct size. Adverse effects of statins seem to be associated with altered cardiomyocyte metabolism. In this review we explore the differences in the mechanism of action and potential side effects of statins in cardiac and skeletal muscle and how they present clinically. These insights may contribute to a more personalized treatment strategy.
Keywords: beneficial and adverse effects; cardiomyocytes; clinical translation; metabolism; statins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ridker P.M., Danielson E., Fonseca F.A.H., Genest J., Gotto A.M., Kastelein J.J.P., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. - DOI - PubMed
-
- Rogers J.K., Jhund P.S., Perez A.C., Bohm M., Cleland J.G., Gullestad L., Kjekshus J., van Veldhuisen D.J., Wikstrand J., Wedel H., et al. Effect of rosuvastatin on repeat heart failure hospitalizations: The CORONA Trial (Controlled Rosuvastatin Multinational Trial in Heart Failure) JACC Heart Fail. 2014;2:289–297. doi: 10.1016/j.jchf.2013.12.007. - DOI - PubMed
-
- Yoshimura S., Uchida K., Daimon T., Takashima R., Kimura K., Morimoto T., Tanada S., Iida T., Kuroda J., Nose A., et al. Randomized Controlled Trial of Early Versus Delayed Statin Therapy in Patients With Acute Ischemic Stroke. Stroke. 2017;48:3057–3063. doi: 10.1161/STROKEAHA.117.017623. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

