Hst1/Gel-MA Scaffold Significantly Promotes the Quality of Osteochondral Regeneration in the Temporomandibular Joint
- PMID: 37888178
- PMCID: PMC10607535
- DOI: 10.3390/jfb14100513
Hst1/Gel-MA Scaffold Significantly Promotes the Quality of Osteochondral Regeneration in the Temporomandibular Joint
Abstract
Objective: Our aim was to evaluate the capacity of the human salivary histatin-1-functionalized methacrylic gelatin scaffold to control osteochondral tissue regeneration and repair in vivo in rabbits with major temporomandibular joint dimensional abnormalities.
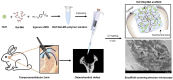
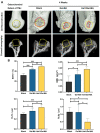
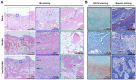
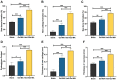
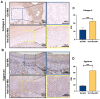
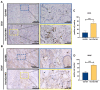
Materials and methods: In order to compare human salivary histatin-1-functionalized methacrylic gelatin scaffolds to the Blank and Gel-MA hydrogel groups, scaffolds were implanted into osteochondral lesions of a critical size (3 × 3 mm) in the anterior region of the condyle of the temporomandibular joint in New Zealand white rabbits. At 4 weeks after implantation, the repair was evaluated using macroscopic examination, histology, and micro-CT analysis.
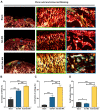
Results: In the comparison of the composite scaffold group with the Blank and Gel-MA groups, analysis of the healed tissue revealed an improved macroscopic appearance in the composite scaffold group. Regeneration was induced by host cell migration in the Hst1/Gel-MA scaffold group.
Conclusions: The current study offers a viable method for in vivo cartilage repair that does not require cell transplantation. Future clinical applications of this strategy's optimization have many potential advantages.
Keywords: cartilage; in vivo; osteochondral; tissue engineering.
Conflict of interest statement
The authors have stated that they have no possible conflicts of interest in relation to the research, writing, or publishing of this paper.
Figures



Similar articles
-
Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints.Pharmaceuticals (Basel). 2021 May 19;14(5):484. doi: 10.3390/ph14050484. Pharmaceuticals (Basel). 2021. PMID: 34069458 Free PMC article.
-
Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits.Acta Biomater. 2016 Mar 1;32:149-160. doi: 10.1016/j.actbio.2015.12.034. Epub 2015 Dec 24. Acta Biomater. 2016. PMID: 26724503
-
Aptamer-Functionalized Bioscaffold Enhances Cartilage Repair by Improving Stem Cell Recruitment in Osteochondral Defects of Rabbit Knees.Am J Sports Med. 2019 Aug;47(10):2316-2326. doi: 10.1177/0363546519856355. Epub 2019 Jun 24. Am J Sports Med. 2019. PMID: 31233332
-
Repair and regeneration of osteochondral defects in the articular joints.Biomol Eng. 2007 Nov;24(5):489-95. doi: 10.1016/j.bioeng.2007.07.014. Epub 2007 Aug 7. Biomol Eng. 2007. PMID: 17931965 Review.
-
Effect of porosities of bilayered porous scaffolds on spontaneous osteochondral repair in cartilage tissue engineering.Regen Biomater. 2015 Mar;2(1):9-19. doi: 10.1093/rb/rbv001. Epub 2015 Mar 6. Regen Biomater. 2015. PMID: 26813511 Free PMC article. Review.
Cited by
-
Histatins, proangiogenic molecules with therapeutic implications in regenerative medicine.iScience. 2024 Nov 1;27(12):111309. doi: 10.1016/j.isci.2024.111309. eCollection 2024 Dec 20. iScience. 2024. PMID: 39634559 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

