The Preparation and Clinical Efficacy of Amnion-Derived Membranes: A Review
- PMID: 37888195
- PMCID: PMC10607219
- DOI: 10.3390/jfb14100531
The Preparation and Clinical Efficacy of Amnion-Derived Membranes: A Review
Abstract
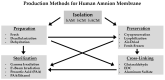
Biological tissues from various anatomical sources have been utilized for tissue transplantation and have developed into an important source of extracellular scaffolding material for regenerative medicine applications. Tissue scaffolds ideally integrate with host tissue and provide a homeostatic environment for cellular infiltration, growth, differentiation, and tissue resolution. The human amniotic membrane is considered an important source of scaffolding material due to its 3D structural architecture and function and as a source of growth factors and cytokines. This tissue source has been widely studied and used in various areas of tissue repair including intraoral reconstruction, corneal repair, tendon repair, microvascular reconstruction, nerve procedures, burns, and chronic wound treatment. The production of amniotic membrane allografts has not been standardized, resulting in a wide array of amniotic membrane products, including single, dual, and tri-layered products, such as amnion, chorion, amnion-chorion, amnion-amnion, and amnion-chorion-amnion allografts. Since these allografts are not processed using the same methods, they do not necessarily produce the same clinical responses. The aim of this review is to highlight the properties of different human allograft membranes, present the different processing and preservation methods, and discuss their use in tissue engineering and regenerative applications.
Keywords: amnion; amnion–chorion; anti-inflammatory; chorion; chronic wound; clinical application; human amniotic membrane; processing; regenerative medicine; skin substitute.
Conflict of interest statement
A.L.I., R.G.A. and A.J.T. are employees of the Carmell Corporation, formerly Axolotl Biologix.
Figures






References
-
- Jay R.M., Huish J.P., Wray J.H. Amniotic membrane in clinical medicine: History, current status, and future use. In: Mooradian D.L., editor. Extracellu-lar Matrix-Derived Implants in Clinical Medicine. 1st ed. Elsevier Inc.; Amsterdam, The Netherlands: 2016. pp. 151–176. - DOI
Publication types
LinkOut - more resources
Full Text Sources

